Differential regulation of cell death programs in males and females by Poly (ADP-Ribose) Polymerase-1 and 17β estradiol
- PMID: 23928697
- PMCID: PMC3763428
- DOI: 10.1038/cddis.2013.251
Differential regulation of cell death programs in males and females by Poly (ADP-Ribose) Polymerase-1 and 17β estradiol
Abstract
Cell death can be divided into the anti-inflammatory process of apoptosis and the pro-inflammatory process of necrosis. Necrosis, as apoptosis, is a regulated form of cell death, and Poly-(ADP-Ribose) Polymerase-1 (PARP-1) and Receptor-Interacting Protein (RIP) 1/3 are major mediators. We previously showed that absence or inhibition of PARP-1 protects mice from nephritis, however only the male mice. We therefore hypothesized that there is an inherent difference in the cell death program between the sexes. We show here that in an immune-mediated nephritis model, female mice show increased apoptosis compared to male mice. Treatment of the male mice with estrogens induced apoptosis to levels similar to that in female mice and inhibited necrosis. Although PARP-1 was activated in both male and female mice, PARP-1 inhibition reduced necrosis only in the male mice. We also show that deletion of RIP-3 did not have a sex bias. We demonstrate here that male and female mice are prone to different types of cell death. Our data also suggest that estrogens and PARP-1 are two of the mediators of the sex-bias in cell death. We therefore propose that targeting cell death based on sex will lead to tailored and better treatments for each gender.
Figures


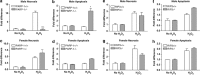
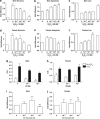
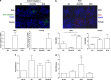
Comment in
-
Taking into account the gender issue in cell death studies.Cell Death Dis. 2014 Mar 13;5(3):e1121. doi: 10.1038/cddis.2014.73. Cell Death Dis. 2014. PMID: 24625980 Free PMC article. No abstract available.
Similar articles
-
Poly(ADP-ribose) polymerase-1 regulates the progression of autoimmune nephritis in males by inducing necrotic cell death and modulating inflammation.J Immunol. 2009 Jun 1;182(11):7297-306. doi: 10.4049/jimmunol.0803565. J Immunol. 2009. PMID: 19454727 Free PMC article.
-
Sex differences in the response to poly(ADP-ribose) polymerase-1 deletion and caspase inhibition after stroke.Stroke. 2011 Apr;42(4):1090-6. doi: 10.1161/STROKEAHA.110.594861. Epub 2011 Feb 10. Stroke. 2011. PMID: 21311064 Free PMC article.
-
Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection.J Cereb Blood Flow Metab. 2005 Apr;25(4):502-12. doi: 10.1038/sj.jcbfm.9600059. J Cereb Blood Flow Metab. 2005. PMID: 15689952
-
Mediation of cell death by poly(ADP-ribose) polymerase-1.Pharmacol Res. 2005 Jul;52(1):5-14. doi: 10.1016/j.phrs.2005.02.011. Pharmacol Res. 2005. PMID: 15911329 Review.
-
Structure and function of poly(ADP-ribose) polymerase-1: role in oxidative stress-related pathologies.Curr Vasc Pharmacol. 2005 Jul;3(3):209-14. doi: 10.2174/1570161054368625. Curr Vasc Pharmacol. 2005. PMID: 16026317 Review.
Cited by
-
Sex differences in apoptosis do not contribute to sex differences in blood pressure or renal T cells in spontaneously hypertensive rats.Front Physiol. 2022 Oct 11;13:1006951. doi: 10.3389/fphys.2022.1006951. eCollection 2022. Front Physiol. 2022. PMID: 36304583 Free PMC article.
-
Sex-specific stress response and HMGB1 release in pulmonary endothelial cells.PLoS One. 2020 Apr 9;15(4):e0231267. doi: 10.1371/journal.pone.0231267. eCollection 2020. PLoS One. 2020. PMID: 32271800 Free PMC article.
-
Inhibition of neuronal ferroptosis protects hemorrhagic brain.JCI Insight. 2017 Apr 6;2(7):e90777. doi: 10.1172/jci.insight.90777. JCI Insight. 2017. PMID: 28405617 Free PMC article.
-
PARP inhibitors protect against sex- and AAG-dependent alkylation-induced neural degeneration.Oncotarget. 2017 Aug 3;8(40):68707-68720. doi: 10.18632/oncotarget.19844. eCollection 2017 Sep 15. Oncotarget. 2017. PMID: 28978150 Free PMC article.
-
Impact of the X Chromosome and sex on regulatory variation.Genome Res. 2016 Jun;26(6):768-77. doi: 10.1101/gr.197897.115. Epub 2016 Apr 21. Genome Res. 2016. PMID: 27197214 Free PMC article.
References
-
- Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. J Autoimmun. 2007;28:1–6. - PubMed
-
- Klein SL. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26:247–264. - PubMed
-
- Cihakova D, Talor MV, Barin JG, Baldeviano GC, Fairweather D, Rose NR, et al. Sex differences in a murine model of Sjogren's syndrome. Ann NY Acad Sci. 2009;1173:378–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

