Subviral dense bodies of human cytomegalovirus stimulate maturation and activation of monocyte-derived immature dendritic cells
- PMID: 23926346
- PMCID: PMC3807270
- DOI: 10.1128/JVI.01429-13
Subviral dense bodies of human cytomegalovirus stimulate maturation and activation of monocyte-derived immature dendritic cells
Abstract
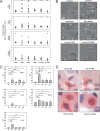
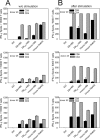
Dendritic cells play a central role in the immune control of human cytomegalovirus (HCMV) infection. This work aimed at investigating the impact of noninfectious, subviral dense bodies of HCMV on the maturation and activation of dendritic cells (DC). Treatment of immature DC with dense bodies led to the maturation of these cells and significantly increased their capacity for cytokine release and antigen presentation. Dense body-activated DC may thereby contribute to the development of antiviral immunity.
Figures
Similar articles
-
Cross-presentation of human cytomegalovirus pp65 (UL83) to CD8+ T cells is regulated by virus-induced, soluble-mediator-dependent maturation of dendritic cells.J Virol. 2002 Jan;76(1):142-50. doi: 10.1128/jvi.76.1.142-150.2002. J Virol. 2002. PMID: 11739680 Free PMC article.
-
Human cytomegalovirus inhibits the migration of immature dendritic cells by down-regulating cell-surface CCR1 and CCR5.J Leukoc Biol. 2005 Feb;77(2):219-28. doi: 10.1189/jlb.0504301. Epub 2004 Nov 2. J Leukoc Biol. 2005. PMID: 15522919
-
Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells.Blood. 2002 Apr 15;99(8):2913-21. doi: 10.1182/blood.v99.8.2913. Blood. 2002. PMID: 11929782
-
Dendritic cells and HCMV cross-presentation.Curr Top Microbiol Immunol. 2003;276:277-94. doi: 10.1007/978-3-662-06508-2_13. Curr Top Microbiol Immunol. 2003. PMID: 12797453 Review.
-
Dendritic cells in cytomegalovirus infection: viral evasion and host countermeasures.APMIS. 2009 May;117(5-6):413-26. doi: 10.1111/j.1600-0463.2009.02449.x. APMIS. 2009. PMID: 19400865 Review.
Cited by
-
Therapeutic Vaccination of Hematopoietic Cell Transplantation Recipients Improves Protective CD8 T-Cell Immunotherapy of Cytomegalovirus Infection.Front Immunol. 2021 Aug 19;12:694588. doi: 10.3389/fimmu.2021.694588. eCollection 2021. Front Immunol. 2021. PMID: 34489940 Free PMC article.
-
Human Cytomegalovirus Primary Infection and Reactivation: Insights From Virion-Carried Molecules.Front Microbiol. 2020 Jul 14;11:1511. doi: 10.3389/fmicb.2020.01511. eCollection 2020. Front Microbiol. 2020. PMID: 32765441 Free PMC article. Review.
-
Identification and Characterization of Epithelial Cell-Derived Dense Bodies Produced upon Cytomegalovirus Infection.Vaccines (Basel). 2022 Aug 12;10(8):1308. doi: 10.3390/vaccines10081308. Vaccines (Basel). 2022. PMID: 36016196 Free PMC article.
-
Cell-mediated immunity to human CMV infection: a brief overview.F1000Prime Rep. 2014 May 6;6:28. doi: 10.12703/P6-28. eCollection 2014. F1000Prime Rep. 2014. PMID: 24860650 Free PMC article. Review.
-
Production Strategies for Pentamer-Positive Subviral Dense Bodies as a Safe Human Cytomegalovirus Vaccine.Vaccines (Basel). 2019 Sep 1;7(3):104. doi: 10.3390/vaccines7030104. Vaccines (Basel). 2019. PMID: 31480520 Free PMC article.
References
-
- Riegler S, Hebart H, Einsele H, Brossart P, Jahn G, Sinzger C. 2000. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Gen. Virol. 81:393–399. - PubMed
-
- Gerna G, Percivalle E, Lilleri D, Lozza L, Fornara C, Hahn G, Baldanti F, Revello MG. 2005. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131–128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J. Gen. Virol. 86:275–284. - PubMed
-
- Raftery MJ, Schwab M, Eibert SM, Samstag Y, Walczak H, Schönrich G. 2001. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15:997–1009. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

