Combination of antibody that inhibits ligand-independent HER3 dimerization and a p110α inhibitor potently blocks PI3K signaling and growth of HER2+ breast cancers
- PMID: 23918797
- PMCID: PMC3790862
- DOI: 10.1158/0008-5472.CAN-13-1191
Combination of antibody that inhibits ligand-independent HER3 dimerization and a p110α inhibitor potently blocks PI3K signaling and growth of HER2+ breast cancers
Abstract
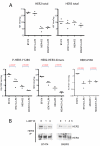
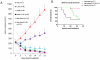
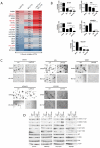
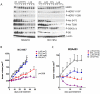
We examined the effects of LJM716, an HER3 (ERBB3) neutralizing antibody that inhibits ligand-induced and ligand-independent HER3 dimerization, as a single agent and in combination with BYL719, an ATP competitive p110α-specific inhibitor, against HER2-overexpressing breast and gastric cancers. Treatment with LJM716 reduced HER2-HER3 and HER3-p85 dimers, P-HER3 and P-AKT, both in vitro and in vivo. Treatment with LJM716 alone markedly reduced growth of BT474 xenografts. The combination of LJM716/lapatinib/trastuzumab significantly improved survival of mice with BT474 xenografts compared with lapatinib/trastuzumab (P = 0.0012). LJM716 and BYL719 synergistically inhibited growth in a panel of HER2+ and PIK3CA mutant cell lines. The combination also inhibited P-AKT in HER2-overexpressing breast cancer cells and growth of HER2+ NCI-N87 gastric cancer xenografts more potently than LJM716 or BYL719 alone. Trastuzumab-resistant HER2+/PIK3CA mutant MDA453 xenografts regressed completely after 3 weeks of therapy with LJM716 and BYL719, whereas either single agent inhibited growth only partially. Finally, mice with BT474 xenografts treated with trastuzumab/LJM716, trastuzumab/BYL719, LJM716/BYL719, or trastuzumab/LJM716/BYL719 exhibited similar rates of tumor regression after 3 weeks of treatment. Thirty weeks after treatment discontinuation, 14% of mice were treated with trastuzumab/LJM716/BYL719, whereas >80% in all other treatment groups were sacrificed due to a recurrent large tumor burden (P = 0.0066). These data suggest that dual blockade of the HER2 signaling network with an HER3 antibody that inhibits HER2-HER3 dimers in combination with a p110α-specific inhibitor in the absence of a direct HER2 antagonist is an effective treatment approach against HER2-overexpressing cancers.
Figures


Similar articles
-
An antibody that locks HER3 in the inactive conformation inhibits tumor growth driven by HER2 or neuregulin.Cancer Res. 2013 Oct 1;73(19):6024-35. doi: 10.1158/0008-5472.CAN-13-1198. Epub 2013 Aug 8. Cancer Res. 2013. PMID: 23928993 Free PMC article.
-
Dual blockade of HER2 in HER2-overexpressing tumor cells does not completely eliminate HER3 function.Clin Cancer Res. 2013 Feb 1;19(3):610-9. doi: 10.1158/1078-0432.CCR-12-2024. Epub 2012 Dec 5. Clin Cancer Res. 2013. PMID: 23224399 Free PMC article.
-
An ERBB1-3 Neutralizing Antibody Mixture With High Activity Against Drug-Resistant HER2+ Breast Cancers With ERBB Ligand Overexpression.J Natl Cancer Inst. 2017 Nov 1;109(11):djx065. doi: 10.1093/jnci/djx065. J Natl Cancer Inst. 2017. PMID: 29059433 Free PMC article.
-
Lapatinib.Recent Results Cancer Res. 2018;211:19-44. doi: 10.1007/978-3-319-91442-8_2. Recent Results Cancer Res. 2018. PMID: 30069757 Review.
-
De-escalation of treatment in HER2-positive breast cancer: Determinants of response and mechanisms of resistance.Breast. 2017 Aug;34 Suppl 1(Suppl 1):S19-S26. doi: 10.1016/j.breast.2017.06.022. Epub 2017 Jul 4. Breast. 2017. PMID: 28687441 Free PMC article. Review.
Cited by
-
Fibroblast-derived neuregulin 1 promotes compensatory ErbB3 receptor signaling in mutant BRAF melanoma.J Biol Chem. 2015 Oct 2;290(40):24267-77. doi: 10.1074/jbc.M115.657270. Epub 2015 Aug 12. J Biol Chem. 2015. PMID: 26269601 Free PMC article.
-
The Role of PI3K/Akt/mTOR Signaling in Gastric Carcinoma.Cancers (Basel). 2014 Jul 7;6(3):1441-63. doi: 10.3390/cancers6031441. Cancers (Basel). 2014. PMID: 25003395 Free PMC article.
-
Measurement of PIP3 levels reveals an unexpected role for p110β in early adaptive responses to p110α-specific inhibitors in luminal breast cancer.Cancer Cell. 2015 Jan 12;27(1):97-108. doi: 10.1016/j.ccell.2014.11.007. Epub 2014 Dec 24. Cancer Cell. 2015. PMID: 25544637 Free PMC article.
-
ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics.Cancer Cell. 2014 Mar 17;25(3):282-303. doi: 10.1016/j.ccr.2014.02.025. Cancer Cell. 2014. PMID: 24651011 Free PMC article. Review.
-
PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting.Nat Rev Cancer. 2015 Jan;15(1):7-24. doi: 10.1038/nrc3860. Nat Rev Cancer. 2015. PMID: 25533673 Free PMC article. Review.
References
-
- Brugge J, Hung MC, Mills GB. A new mutational AKTivation in the PI3K pathway. Cancer Cell. 2007;12:104–7. - PubMed
-
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. - PubMed
-
- Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–7. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. - PubMed
-
- Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16:413–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

