RIP3: a molecular switch for necrosis and inflammation
- PMID: 23913919
- PMCID: PMC3744722
- DOI: 10.1101/gad.223321.113
RIP3: a molecular switch for necrosis and inflammation
Abstract
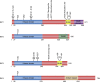
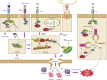
The receptor-interacting protein kinase 3 (RIP3/RIPK3) has emerged as a critical regulator of programmed necrosis/necroptosis, an inflammatory form of cell death with important functions in pathogen-induced and sterile inflammation. RIP3 activation is tightly regulated by phosphorylation, ubiquitination, and caspase-mediated cleavage. These post-translational modifications coordinately regulate the assembly of a macromolecular signaling complex termed the necrosome. Recently, several reports indicate that RIP3 can promote inflammation independent of its pronecrotic activity. Here, we review our current understanding of the mechanisms that drive RIP3-dependent necrosis and its role in different inflammatory diseases.
Keywords: FADD; MLKL; PGAM5; RIP1; caspase 8; inflammation.
Figures
Similar articles
-
CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis.PLoS One. 2013 Oct 2;8(10):e76841. doi: 10.1371/journal.pone.0076841. eCollection 2013. PLoS One. 2013. PMID: 24098568 Free PMC article.
-
Necroptosis in health and diseases.Semin Cell Dev Biol. 2014 Nov;35:14-23. doi: 10.1016/j.semcdb.2014.07.013. Epub 2014 Aug 1. Semin Cell Dev Biol. 2014. PMID: 25087983 Review.
-
Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL.J Biol Chem. 2013 Oct 25;288(43):31268-79. doi: 10.1074/jbc.M113.462341. Epub 2013 Sep 9. J Biol Chem. 2013. PMID: 24019532 Free PMC article.
-
PI3K mediates tumor necrosis factor induced-necroptosis through initiating RIP1-RIP3-MLKL signaling pathway activation.Cytokine. 2020 May;129:155046. doi: 10.1016/j.cyto.2020.155046. Epub 2020 Feb 28. Cytokine. 2020. PMID: 32114297
-
The regulation of necroptosis by post-translational modifications.Cell Death Differ. 2021 Mar;28(3):861-883. doi: 10.1038/s41418-020-00722-7. Epub 2021 Jan 18. Cell Death Differ. 2021. PMID: 33462412 Free PMC article. Review.
Cited by
-
Toxicity of Necrostatin-1 in Parkinson's Disease Models.Antioxidants (Basel). 2020 Jun 15;9(6):524. doi: 10.3390/antiox9060524. Antioxidants (Basel). 2020. PMID: 32549347 Free PMC article.
-
Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death.Cell Death Dis. 2015 Feb 12;6(2):e1636. doi: 10.1038/cddis.2015.16. Cell Death Dis. 2015. PMID: 25675296 Free PMC article.
-
Glutaminolysis and Transferrin Regulate Ferroptosis.Mol Cell. 2015 Jul 16;59(2):298-308. doi: 10.1016/j.molcel.2015.06.011. Epub 2015 Jul 9. Mol Cell. 2015. PMID: 26166707 Free PMC article.
-
Necroptosis suppresses inflammation via termination of TNF- or LPS-induced cytokine and chemokine production.Cell Death Differ. 2015 Aug;22(8):1313-27. doi: 10.1038/cdd.2014.222. Epub 2015 Jan 23. Cell Death Differ. 2015. PMID: 25613374 Free PMC article.
-
Regulated necrosis, a proinflammatory cell death, potentially counteracts pathogenic infections.Cell Death Dis. 2022 Jul 22;13(7):637. doi: 10.1038/s41419-022-05066-3. Cell Death Dis. 2022. PMID: 35869043 Free PMC article. Review.
References
-
- Bhatia M, He M, Zhang H, Moochhala S 2009. Sepsis as a model of SIRS. Front Biosci 14: 4703–4711 - PubMed
-
- Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M 2011. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity 35: 572–582 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
