A discoidin domain receptor 1 knock-out mouse as a novel model for osteoarthritis of the temporomandibular joint
- PMID: 23912900
- PMCID: PMC11113511
- DOI: 10.1007/s00018-013-1436-8
A discoidin domain receptor 1 knock-out mouse as a novel model for osteoarthritis of the temporomandibular joint
Abstract
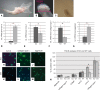
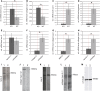
Discoidin domain receptor 1 (DDR-1)-deficient mice exhibited a high incidence of osteoarthritis (OA) in the temporomandibular joint (TMJ) as early as 9 weeks of age. They showed typical histological signs of OA, including surface fissures, loss of proteoglycans, chondrocyte cluster formation, collagen type I upregulation, and atypical collagen fibril arrangements. Chondrocytes isolated from the TMJs of DDR-1-deficient mice maintained their osteoarthritic characteristics when placed in culture. They expressed high levels of runx-2 and collagen type I, as well as low levels of sox-9 and aggrecan. The expression of DDR-2, a key factor in OA, was increased. DDR-1-deficient chondrocytes from the TMJ were positively influenced towards chondrogenesis by a three-dimensional matrix combined with a runx-2 knockdown or stimulation with extracellular matrix components, such as nidogen-2. Therefore, the DDR-1 knock-out mouse can serve as a novel model for temporomandibular disorders, such as OA of the TMJ, and will help to develop new treatment options, particularly those involving tissue regeneration.
Figures





Similar articles
-
Pathological mechanism of chondrocytes and the surrounding environment during osteoarthritis of temporomandibular joint.J Cell Mol Med. 2021 Jun;25(11):4902-4911. doi: 10.1111/jcmm.16514. Epub 2021 May 5. J Cell Mol Med. 2021. PMID: 33949768 Free PMC article. Review.
-
Biglycan and fibromodulin have essential roles in regulating chondrogenesis and extracellular matrix turnover in temporomandibular joint osteoarthritis.Am J Pathol. 2010 Feb;176(2):812-26. doi: 10.2353/ajpath.2010.090450. Epub 2009 Dec 24. Am J Pathol. 2010. PMID: 20035055 Free PMC article.
-
Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis.Arthritis Rheum. 2007 Aug;56(8):2663-73. doi: 10.1002/art.22761. Arthritis Rheum. 2007. PMID: 17665456
-
Delayed progression of condylar cartilage degeneration, by reduction of the discoidin domain receptor 2, in the temporomandibular joints of osteoarthritic mouse models.J Oral Pathol Med. 2014 Apr;43(4):317-21. doi: 10.1111/jop.12137. J Oral Pathol Med. 2014. PMID: 24822272 Free PMC article.
-
Osteophyte formation and matrix mineralization in a TMJ osteoarthritis mouse model are associated with ectopic hedgehog signaling.Matrix Biol. 2016 May-Jul;52-54:339-354. doi: 10.1016/j.matbio.2016.03.001. Epub 2016 Mar 3. Matrix Biol. 2016. PMID: 26945615 Free PMC article. Review.
Cited by
-
Discoidin domain receptors in disease.Matrix Biol. 2014 Feb;34:185-92. doi: 10.1016/j.matbio.2013.12.002. Epub 2013 Dec 19. Matrix Biol. 2014. PMID: 24361528 Free PMC article. Review.
-
The degeneration-pain relationship in the temporomandibular joint: Current understandings and rodent models.Front Pain Res (Lausanne). 2023 Feb 9;4:1038808. doi: 10.3389/fpain.2023.1038808. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 36846071 Free PMC article. Review.
-
Collagen advanced glycation inhibits its Discoidin Domain Receptor 2 (DDR2)-mediated induction of lysyl oxidase in osteoblasts.Bone. 2014 Jan;58:33-41. doi: 10.1016/j.bone.2013.10.001. Epub 2013 Oct 10. Bone. 2014. PMID: 24120383 Free PMC article.
-
Lubricin is Required for the Structural Integrity and Post-natal Maintenance of TMJ.J Dent Res. 2014 Jul;93(7):663-70. doi: 10.1177/0022034514535807. Epub 2014 May 16. J Dent Res. 2014. PMID: 24834922 Free PMC article.
-
Pathological mechanism of chondrocytes and the surrounding environment during osteoarthritis of temporomandibular joint.J Cell Mol Med. 2021 Jun;25(11):4902-4911. doi: 10.1111/jcmm.16514. Epub 2021 May 5. J Cell Mol Med. 2021. PMID: 33949768 Free PMC article. Review.
References
-
- Carlson GE, Magnusson T. Management of temporomandibular disorders in the general dental practice. Berlin: Quintessenz; 1999.
-
- Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. - PubMed
-
- Horton WE, Jr, Bennion P, Yang L. Cellular, molecular, and matrix changes in cartilage during aging and osteoarthritis. J Musculoskel Neuronal Interact. 2006;6(4):379–381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

