Stromal disrupting effects of nab-paclitaxel in pancreatic cancer
- PMID: 23907428
- PMCID: PMC3749580
- DOI: 10.1038/bjc.2013.415
Stromal disrupting effects of nab-paclitaxel in pancreatic cancer
Abstract
Background: Nab-paclitaxel and gemcitabine have demonstrated a survival benefit over gemcitabine alone in advanced pancreatic cancer (PDA). This study aimed to investigate the clinical, biological, and imaging effects of the regimen in patients with operable PDA.
Methods: Patients with operable PDA received two cycles of nab-paclitaxel and gemcitabine before surgical resection. FDG-PET and CA19.9 tumour marker levels were used to measure clinical activity. Effects on tumour stroma were determined by endoscopic ultrasound (EUS) elastography. The collagen content and architecture as well as density of cancer-associated fibroblasts (CAFs) were determined in the resected surgical specimen and compared with a group of untreated and treated with conventional chemoradiation therapy controls. A co-clinical study in a mouse model of PDA was conducted to differentiate between the effects of nab-paclitaxel and gemcitabine.
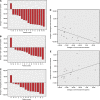
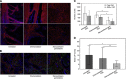
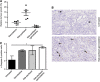
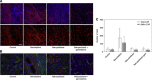
Results: A total of 16 patients were enrolled. Treatment resulted in significant antitumour effects with 50% of patients achieving a >75% decrease in circulating CA19.9 tumour marker and a response by FDG-PET. There was also a significant decrement in tumour stiffness as measured by EUS elastography. Seven of 12 patients who completed treatment and were operated had major pathological regressions. Analysis of residual tumours showed a marked disorganised collagen with a very low density of CAF, which was not observed in the untreated or conventionally treated control groups. The preclinical co-clinical study showed that these effects were specific of nab-paclitaxel and not gemcitabine.
Conclusion: These data suggest that nab-paclitaxel and gemcitabine decreases CAF content inducing a marked alteration in cancer stroma that results in tumour softening. This regimen should be studied in patients with operable PDA.
Figures
Comment in
-
Stellate cells, a point of light in the dark night of pancreatic cancer.Br J Cancer. 2014 Oct 14;111(8):1676-7. doi: 10.1038/bjc.2014.59. Epub 2014 Mar 18. Br J Cancer. 2014. PMID: 24642614 Free PMC article. No abstract available.
-
Neoadjuvant chemotherapy in pancreatic cancer: innovative, but still difficult.Br J Cancer. 2014 Oct 14;111(8):1675-6. doi: 10.1038/bjc.2014.60. Epub 2014 Mar 18. Br J Cancer. 2014. PMID: 24642615 Free PMC article. No abstract available.
-
Reply: 'Comments on Stromal disrupting effects of nab-paclitaxel in pancreatic cancer'.Br J Cancer. 2014 Oct 14;111(8):1677-8. doi: 10.1038/bjc.2014.129. Epub 2014 Mar 18. Br J Cancer. 2014. PMID: 24642623 Free PMC article. No abstract available.
Similar articles
-
Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial.J Clin Oncol. 2011 Dec 1;29(34):4548-54. doi: 10.1200/JCO.2011.36.5742. Epub 2011 Oct 3. J Clin Oncol. 2011. PMID: 21969517 Free PMC article. Clinical Trial.
-
Neoadjuvant Chemotherapy with Gemcitabine Plus Nab-paclitaxel Reduces the Number of Cancer-associated Fibroblasts Through Depletion of Pancreatic Stroma.Anticancer Res. 2018 Jan;38(1):337-343. doi: 10.21873/anticanres.12227. Anticancer Res. 2018. PMID: 29277792
-
Preoperative treatment with gemcitabine plus nab-paclitaxel is a safe and effective chemotherapy for pancreatic adenocarcinoma.Eur J Surg Oncol. 2016 Sep;42(9):1394-400. doi: 10.1016/j.ejso.2016.01.006. Epub 2016 Jan 29. Eur J Surg Oncol. 2016. PMID: 26899943
-
Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies.Cancer Treat Rev. 2014 Feb;40(1):118-28. doi: 10.1016/j.ctrv.2013.04.004. Epub 2013 Jul 9. Cancer Treat Rev. 2014. PMID: 23849556 Review.
-
Treatment of metastatic pancreatic adenocarcinoma: a review.Oncology (Williston Park). 2014 Jan;28(1):70-4. Oncology (Williston Park). 2014. PMID: 24683721 Review.
Cited by
-
Innovative Experimental Ultrasound and US-Related Techniques Using the Murine Model in Pancreatic Ductal Adenocarcinoma: A Systematic Review.J Clin Med. 2023 Dec 14;12(24):7677. doi: 10.3390/jcm12247677. J Clin Med. 2023. PMID: 38137745 Free PMC article. Review.
-
Recent advances in cancer-associated fibroblast: Biomarkers, signaling pathways, and therapeutic opportunities.Chin Med J (Engl). 2024 Mar 20;137(6):638-650. doi: 10.1097/CM9.0000000000003031. Epub 2024 Feb 29. Chin Med J (Engl). 2024. PMID: 38420743 Free PMC article. Review.
-
Neoadjuvant plus adjuvant or only adjuvant nab-paclitaxel plus gemcitabine for resectable pancreatic cancer - the NEONAX trial (AIO-PAK-0313), a prospective, randomized, controlled, phase II study of the AIO pancreatic cancer group.BMC Cancer. 2018 Dec 29;18(1):1298. doi: 10.1186/s12885-018-5183-y. BMC Cancer. 2018. PMID: 30594153 Free PMC article.
-
CAFs and TGF-β Signaling Activation by Mast Cells Contribute to Resistance to Gemcitabine/Nabpaclitaxel in Pancreatic Cancer.Cancers (Basel). 2019 Mar 7;11(3):330. doi: 10.3390/cancers11030330. Cancers (Basel). 2019. PMID: 30866547 Free PMC article.
-
Targeting Tumor-Stromal Interactions in Pancreatic Cancer: Impact of Collagens and Mechanical Traits.Front Cell Dev Biol. 2021 Nov 25;9:787485. doi: 10.3389/fcell.2021.787485. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34901028 Free PMC article. Review.
References
-
- Andriulli A, Festa V, Botteri E, Valvano MR, Koch M, Bassi C, Maisonneuve P, Sebastiano PD. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol. 2012;19:1644–1662. - PubMed
-
- Chun YS, Cooper HS, Cohen SJ, Konski A, Burtness B, Denlinger CS, Astsaturov I, Hall MJ, Hoffman JP. Significance of pathologic response to preoperative therapy in pancreatic cancer. Ann Surg Oncol. 2011;18:3601–3607. - PubMed
-
- Dawwas MF, Taha H, Leeds JS, Nayar MK, Oppong KW. Diagnostic accuracy of quantitative EUS elastography for discriminating malignant from benign solid pancreatic masses: a prospective, single-center study. Gastrointest Endosc. 2012;76 (5:953–961. - PubMed
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

