Rab40b regulates trafficking of MMP2 and MMP9 during invadopodia formation and invasion of breast cancer cells
- PMID: 23902685
- PMCID: PMC3795337
- DOI: 10.1242/jcs.126573
Rab40b regulates trafficking of MMP2 and MMP9 during invadopodia formation and invasion of breast cancer cells
Abstract
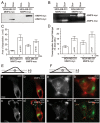
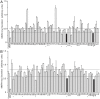
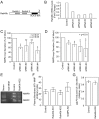
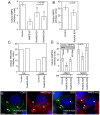
Invadopodia-dependent degradation of the basement membrane plays a major role during metastasis of breast cancer cells. Basement membrane degradation is mediated by targeted secretion of various matrix metalloproteinases (MMPs). Specifically, MMP2 and MMP9 (MMP2/9) possess the ability to hydrolyze components of the basement membrane and regulate various aspects of tumor growth and metastasis. However, the membrane transport machinery that mediates targeting of MMP2/9 to the invadopodia during cancer cell invasion remains to be defined. Because Rab GTPases are key regulators of membrane transport, we screened a human Rab siRNA library and identified Rab40b GTPase as a protein required for secretion of MMP2/9. We also have shown that Rab40b functions during at least two distinct steps of MMP2/9 transport. Here, we demonstrate that Rab40b is required for MMP2/9 sorting into VAMP4-containing secretory vesicles. We also show that Rab40b regulates transport of MMP2/9 secretory vesicles during invadopodia formation and is required for invadopodia-dependent extracellular matrix degradation. Finally, we demonstrate that Rab40b is also required for breast cancer cell invasion in vitro. On the basis of these findings, we propose that Rab40b mediates trafficking of MMP2/9 during invadopodia formation and metastasis of breast cancer cells.
Keywords: Invadopodia; Invasion; MMP2; MMP9; Rab40b; Secretion.
Figures
Similar articles
-
The role and regulation of Rab40b-Tks5 complex during invadopodia formation and cancer cell invasion.J Cell Sci. 2016 Dec 1;129(23):4341-4353. doi: 10.1242/jcs.193904. Epub 2016 Oct 27. J Cell Sci. 2016. PMID: 27789576 Free PMC article.
-
CDCP1 regulates the function of MT1-MMP and invadopodia-mediated invasion of cancer cells.Mol Cancer Res. 2013 Jun;11(6):628-37. doi: 10.1158/1541-7786.MCR-12-0544. Epub 2013 Feb 25. Mol Cancer Res. 2013. PMID: 23439492
-
Rab40b and Tks5 interact at invadopodia during metastasis.J Cell Sci. 2016 Dec 1;129(23):e2301. J Cell Sci. 2016. PMID: 27909076 No abstract available.
-
The regulation of MMP targeting to invadopodia during cancer metastasis.Front Cell Dev Biol. 2015 Feb 2;3:4. doi: 10.3389/fcell.2015.00004. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 25699257 Free PMC article. Review.
-
Targeting SNARE-Mediated Vesicle Transport to Block Invadopodium-Based Cancer Cell Invasion.Front Oncol. 2021 May 21;11:679955. doi: 10.3389/fonc.2021.679955. eCollection 2021. Front Oncol. 2021. PMID: 34094984 Free PMC article. Review.
Cited by
-
MiR-324-5p Suppresses Hepatocellular Carcinoma Cell Invasion by Counteracting ECM Degradation through Post-Transcriptionally Downregulating ETS1 and SP1.PLoS One. 2015 Jul 15;10(7):e0133074. doi: 10.1371/journal.pone.0133074. eCollection 2015. PLoS One. 2015. PMID: 26177288 Free PMC article.
-
Long non-coding RNA highly up-regulated in liver cancer promotes epithelial-to-mesenchymal transition process in oral squamous cell carcinoma.J Cell Mol Med. 2019 Apr;23(4):2645-2655. doi: 10.1111/jcmm.14160. Epub 2019 Jan 24. J Cell Mol Med. 2019. PMID: 30677230 Free PMC article.
-
Global ablation of the mouse Rab11a gene impairs early embryogenesis and matrix metalloproteinase secretion.J Biol Chem. 2014 Nov 14;289(46):32030-32043. doi: 10.1074/jbc.M113.538223. Epub 2014 Sep 30. J Biol Chem. 2014. PMID: 25271168 Free PMC article.
-
Consequences of Rab GTPase dysfunction in genetic or acquired human diseases.Small GTPases. 2018 Mar 4;9(1-2):158-181. doi: 10.1080/21541248.2017.1397833. Epub 2017 Dec 28. Small GTPases. 2018. PMID: 29239692 Free PMC article. Review.
-
Myosin-IIA heavy chain phosphorylation on S1943 regulates tumor metastasis.Exp Cell Res. 2018 Sep 15;370(2):273-282. doi: 10.1016/j.yexcr.2018.06.028. Epub 2018 Jun 25. Exp Cell Res. 2018. PMID: 29953877 Free PMC article.
References
-
- Artym V. V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K. M., Mueller S. C. (2006). Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 66, 3034–3043 10.1158/0008-5472.CAN-05-2177 - DOI - PubMed
-
- Bourguignon L. Y., Gunja-Smith Z., Iida N. (1998). CD44v3,8�–10 is involved in cytoskeleton-mediated tumor cell migration and matrix metalloproteinase (MMP-9) association in metastatic breast cancer cells. J. Cell Physiol. 176, 206–215 - PubMed
-
- Caswell P. T., Spence H. J., Parsons M., White D. P., Clark K., Cheng K. W., Mills G. B., Humphries M. J., Messent A. J., Anderson K. I. et al.(2007). Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev. Cell 13, 496–510 10.1016/j.devcel.2007.08.012 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

