Combining crystallography and EPR: crystal and solution structures of the multidomain cochaperone DnaJ
- PMID: 23897477
- PMCID: PMC3727329
- DOI: 10.1107/S0907444913010640
Combining crystallography and EPR: crystal and solution structures of the multidomain cochaperone DnaJ
Abstract
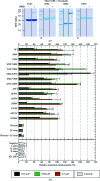
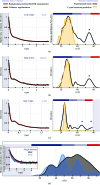
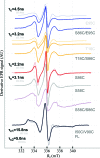
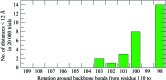
Hsp70 chaperones assist in a large variety of protein-folding processes in the cell. Crucial for these activities is the regulation of Hsp70 by Hsp40 cochaperones. DnaJ, the bacterial homologue of Hsp40, stimulates ATP hydrolysis by DnaK (Hsp70) and thus mediates capture of substrate protein, but is also known to possess chaperone activity of its own. The first structure of a complete functional dimeric DnaJ was determined and the mobility of its individual domains in solution was investigated. Crystal structures of the complete molecular cochaperone DnaJ from Thermus thermophilus comprising the J, GF and C-terminal domains and of the J and GF domains alone showed an ordered GF domain interacting with the J domain. Structure-based EPR spin-labelling studies as well as cross-linking results showed the existence of multiple states of DnaJ in solution with different arrangements of the various domains, which has implications for the function of DnaJ.
Keywords: cross-linking; crystal dehydration; electron paramagnetic resonance; hybrid structure determination; molecular chaperones; radiation-damage-induced phasing with anomalous scattering; single-wavelength anomalous diffraction.
Figures

Similar articles
-
Two J domains ensure high cochaperone activity of DnaJ, Escherichia coli heat shock protein 40.J Biochem. 2018 Aug 1;164(2):153-163. doi: 10.1093/jb/mvy038. J Biochem. 2018. PMID: 29635480
-
Structural features required for the interaction of the Hsp70 molecular chaperone DnaK with its cochaperone DnaJ.J Biol Chem. 1999 Oct 22;274(43):30534-9. doi: 10.1074/jbc.274.43.30534. J Biol Chem. 1999. PMID: 10521435
-
Regulation of ATPase and chaperone cycle of DnaK from Thermus thermophilus by the nucleotide exchange factor GrpE.J Mol Biol. 2001 Feb 2;305(5):1173-83. doi: 10.1006/jmbi.2000.4373. J Mol Biol. 2001. PMID: 11162122
-
The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones.Cell Mol Life Sci. 2006 Nov;63(22):2560-70. doi: 10.1007/s00018-006-6192-6. Cell Mol Life Sci. 2006. PMID: 16952052 Free PMC article. Review.
-
Specification of Hsp70 Function by Hsp40 Co-chaperones.Subcell Biochem. 2023;101:127-139. doi: 10.1007/978-3-031-14740-1_4. Subcell Biochem. 2023. PMID: 36520305 Review.
Cited by
-
Complete loss of the DNAJB6 G/F domain and novel missense mutations cause distal-onset DNAJB6 myopathy.Acta Neuropathol Commun. 2015 Jul 25;3:44. doi: 10.1186/s40478-015-0224-0. Acta Neuropathol Commun. 2015. PMID: 26205529 Free PMC article.
-
The Hsp70-Chaperone Machines in Bacteria.Front Mol Biosci. 2021 Jun 7;8:694012. doi: 10.3389/fmolb.2021.694012. eCollection 2021. Front Mol Biosci. 2021. PMID: 34164436 Free PMC article. Review.
-
X-ray crystallography over the past decade for novel drug discovery - where are we heading next?Expert Opin Drug Discov. 2015;10(9):975-89. doi: 10.1517/17460441.2015.1061991. Epub 2015 Jul 15. Expert Opin Drug Discov. 2015. PMID: 26177814 Free PMC article. Review.
-
The roles of a flagellar HSP40 ensuring rhythmic beating.Mol Biol Cell. 2019 Jan 15;30(2):228-241. doi: 10.1091/mbc.E18-01-0047. Epub 2018 Nov 14. Mol Biol Cell. 2019. PMID: 30427757 Free PMC article.
-
Nematode CDC-37 and DNJ-13 form complexes and can interact with HSP-90.Sci Rep. 2021 Nov 1;11(1):21346. doi: 10.1038/s41598-021-00885-4. Sci Rep. 2021. PMID: 34725424 Free PMC article.
References
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

