Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma
- PMID: 23891972
- PMCID: PMC3896919
- DOI: 10.1053/j.gastro.2013.07.025
Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma
Abstract
Background & aims: Pancreatic ductal adenocarcinoma (PDAC) is characterized by a prominent desmoplastic microenvironment that contains many different immune cells. Activated pancreatic stellate cells (PSCs) contribute to the desmoplasia. We investigated whether distinct stromal compartments are differentially infiltrated by different types of immune cells.
Methods: We used tissue microarray analysis to compare immune cell infiltration of different pancreaticobiliary diseased tissues (PDAC, ampullary carcinoma, cholangiocarcinoma, mucinous cystic neoplasm, chronic inflammation, and chronic pancreatitis) and juxtatumoral stromal (<100 μm from tumor) and panstromal compartments. We investigated the association between immune infiltrate and patient survival times. We also analyzed T-cell migration and tumor infiltration in LSL-KrasG12D/+; LSL-Trp53R172H/+; Pdx-1-Cre (KPC) mice and the effects of all-trans retinoic acid (ATRA) on these processes.
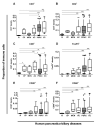
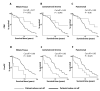
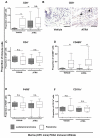
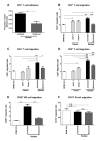
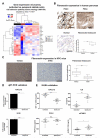
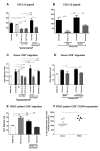
Results: Juxtatumoral compartments in PDAC samples from 2 independent groups of patients contained increased numbers of myeloperoxidase(+) and CD68(+) cells compared with panstromal compartments. However, juxtatumoral compartments of PDACs contained fewer CD8(+), FoxP3(+), CD56(+), or CD20(+) cells than panstromal compartments, a distinction absent in ampullary carcinomas and cholangiocarcinomas. Patients with PDACs that had high densities of CD8(+) T cells in the juxtatumoral compartment had longer survival times than patients with lower densities. In KPC mice, administration of ATRA, which renders PSCs quiescent, increased numbers of CD8(+) T cells in juxtatumoral compartments. We found that activated PSCs express cytokines, chemokines, and adhesion molecules that regulate T-cell migration. In vitro migration assays showed that CD8(+) T cells, from patients with PDAC, had increased chemotaxis toward activated PSCs, which secrete CXCL12, compared with quiescent PSCs or tumor cells. These effects could be reversed by knockdown of CXCL12 or treatment of PSCs with ATRA.
Conclusions: Based on studies of human PDAC samples and KPC mice, activated PSCs appear to reduce migration of CD8(+) T cells to juxtatumoral stromal compartments, preventing their access to cancer cells. Deregulated signaling by activated PSCs could prevent an effective antitumor immune response.
Keywords: AC; ATRA; Antitumor Immunity; CC; Immune Regulation; MDSC; Mouse Model; PBD; PDAC; PSC; Pancreatic Cancer; TMA; Treg; aPSC; activated pancreatic stellate cell; all-trans retinoic acid; ampullary carcinoma; cholangiocarcinoma; myeloid-derived suppressor cell; pancreatic ductal adenocarcinoma; pancreatic stellate cell; pancreaticobiliary disease; qPSC; quiescent pancreatic stellate cell; regulatory T cell; tissue microarray.
Copyright © 2013 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression.Gastroenterology. 2011 Oct;141(4):1486-97, 1497.e1-14. doi: 10.1053/j.gastro.2011.06.047. Epub 2011 Jun 24. Gastroenterology. 2011. PMID: 21704588
-
NFκB in Pancreatic Stellate Cells Reduces Infiltration of Tumors by Cytotoxic T Cells and Killing of Cancer Cells, via Up-regulation of CXCL12.Gastroenterology. 2018 Sep;155(3):880-891.e8. doi: 10.1053/j.gastro.2018.05.051. Epub 2018 Jul 29. Gastroenterology. 2018. PMID: 29909021 Free PMC article.
-
Pancreatic stellate cell secreted IL-6 stimulates STAT3 dependent invasiveness of pancreatic intraepithelial neoplasia and cancer cells.Oncotarget. 2016 Oct 4;7(40):65982-65992. doi: 10.18632/oncotarget.11786. Oncotarget. 2016. PMID: 27602757 Free PMC article.
-
Pancreatic stellate cells and pancreas cancer: current perspectives and future strategies.Eur J Cancer. 2014 Oct;50(15):2570-82. doi: 10.1016/j.ejca.2014.06.021. Epub 2014 Aug 1. Eur J Cancer. 2014. PMID: 25091797 Review.
-
Pancreatic stellate cell: Pandora's box for pancreatic disease biology.World J Gastroenterol. 2017 Jan 21;23(3):382-405. doi: 10.3748/wjg.v23.i3.382. World J Gastroenterol. 2017. PMID: 28210075 Free PMC article. Review.
Cited by
-
The Immune Microenvironment in Pancreatic Cancer.Int J Mol Sci. 2020 Oct 3;21(19):7307. doi: 10.3390/ijms21197307. Int J Mol Sci. 2020. PMID: 33022971 Free PMC article. Review.
-
Exploring the Biology of Cancer-Associated Fibroblasts in Pancreatic Cancer.Cancers (Basel). 2022 Oct 28;14(21):5302. doi: 10.3390/cancers14215302. Cancers (Basel). 2022. PMID: 36358721 Free PMC article. Review.
-
Prognostic value of tertiary lymphoid structures (TLS) in digestive system cancers: a systematic review and meta-analysis.BMC Cancer. 2023 Dec 18;23(1):1248. doi: 10.1186/s12885-023-11738-w. BMC Cancer. 2023. PMID: 38110876 Free PMC article.
-
Targeting myeloid suppressive cells revives cytotoxic anti-tumor responses in pancreatic cancer.iScience. 2022 Oct 9;25(11):105317. doi: 10.1016/j.isci.2022.105317. eCollection 2022 Nov 18. iScience. 2022. PMID: 36310582 Free PMC article.
-
The Heterogeneity of the Tumor Microenvironment as Essential Determinant of Development, Progression and Therapy Response of Pancreatic Cancer.Cancers (Basel). 2021 Sep 30;13(19):4932. doi: 10.3390/cancers13194932. Cancers (Basel). 2021. PMID: 34638420 Free PMC article. Review.
References
-
- Perez-Mancera PA, Guerra C, Barbacid M, et al. What We Have Learned About Pancreatic Cancer from Mouse Models. Gastroenterology. 2012 - PubMed
-
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. - PubMed
-
- Vesely MD, Kershaw MH, Schreiber RD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

