Regimen-specific effects of RNA-modified chimeric antigen receptor T cells in mice with advanced leukemia
- PMID: 23883116
- PMCID: PMC3746289
- DOI: 10.1089/hum.2013.075
Regimen-specific effects of RNA-modified chimeric antigen receptor T cells in mice with advanced leukemia
Abstract
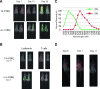
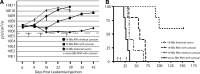
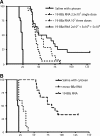
Cytotoxic T lymphocytes modified with chimeric antigen receptors (CARs) for adoptive immunotherapy of hematologic malignancies have demonstrated activity in early phase clinical trials. While T cells bearing stably expressed CARs are efficacious and have potential long-term persistence, temporary expression of a CAR via RNA electroporation is also potentially efficacious in preclinical models. Temporary CAR expression using RNA presents a method of testing CARs clinically with additional safety where there may be concerns about possible chronic "on-target, off-tumor" toxic effects, as the degradation of RNA ensures complete removal of the CAR over time without relying on suicide induction systems. CD19-directed RNA CAR T cells were tested in vivo for efficacy and comparison to lentiviral vector (LV)-generated stable CAR T cells. We tested the hypothesis that multiple infusions of RNA CAR T cells preceded by lymphodepleting chemotherapy could mediate improved survival and sustained antitumor responses in a robust leukemia xenograft model. The saturation strategy using rationally designed multiple infusions of RNA CARs based on multiple model iterations approached the efficacy of a stable LV expression method. Two-color imaging revealed that relapse was a locoregional phenomenon in both the temporary and the stable expression models. In marked contrast to stably expressed CARs with retroviral or LV technology, the efficacy of RNA CARs appears independent of the costimulatory signaling endodomains likely because they more influence proliferation and persistence rather than short-term efficacy. The efficacy of the RNA CAR infusions may approach that of stably expressed CARs, offer theoretically safer initial clinical testing in addition to suicide systems, and allow for rapid and effective iterative preclinical modeling for the testing of new targets.
Figures


Similar articles
-
Treatment of advanced leukemia in mice with mRNA engineered T cells.Hum Gene Ther. 2011 Dec;22(12):1575-86. doi: 10.1089/hum.2011.070. Epub 2011 Sep 23. Hum Gene Ther. 2011. PMID: 21838572 Free PMC article.
-
A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines.J Immunother Cancer. 2017 May 16;5:42. doi: 10.1186/s40425-017-0246-1. eCollection 2017. J Immunother Cancer. 2017. PMID: 28515942 Free PMC article.
-
Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors.Nat Rev Clin Oncol. 2013 May;10(5):267-76. doi: 10.1038/nrclinonc.2013.46. Epub 2013 Apr 2. Nat Rev Clin Oncol. 2013. PMID: 23546520 Free PMC article. Review.
-
Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo.Mol Ther. 2009 Aug;17(8):1453-64. doi: 10.1038/mt.2009.83. Epub 2009 Apr 21. Mol Ther. 2009. PMID: 19384291 Free PMC article.
-
Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy.Clin Cancer Res. 2016 Apr 15;22(8):1875-84. doi: 10.1158/1078-0432.CCR-15-1433. Clin Cancer Res. 2016. PMID: 27084741 Free PMC article. Review.
Cited by
-
Advancements in Personalized CAR-T Therapy: Comprehensive Overview of Biomarkers and Therapeutic Targets in Hematological Malignancies.Int J Mol Sci. 2024 Jul 15;25(14):7743. doi: 10.3390/ijms25147743. Int J Mol Sci. 2024. PMID: 39062986 Free PMC article. Review.
-
Chimeric antigen receptor-modified T cells for the treatment of solid tumors: Defining the challenges and next steps.Pharmacol Ther. 2016 Oct;166:30-9. doi: 10.1016/j.pharmthera.2016.06.010. Epub 2016 Jun 29. Pharmacol Ther. 2016. PMID: 27373504 Free PMC article. Review.
-
Novel anti-CD3 chimeric antigen receptor targeting of aggressive T cell malignancies.Oncotarget. 2016 Aug 30;7(35):56219-56232. doi: 10.18632/oncotarget.11019. Oncotarget. 2016. PMID: 27494836 Free PMC article.
-
The biological basis and clinical symptoms of CAR-T therapy-associated toxicites.Cell Death Dis. 2018 Sep 4;9(9):897. doi: 10.1038/s41419-018-0918-x. Cell Death Dis. 2018. PMID: 30181581 Free PMC article. Review.
-
Emerging Cellular Therapies for Cancer.Annu Rev Immunol. 2019 Apr 26;37:145-171. doi: 10.1146/annurev-immunol-042718-041407. Epub 2018 Dec 10. Annu Rev Immunol. 2019. PMID: 30526160 Free PMC article. Review.
References
-
- Bleyer W.A. Cancer chemotherapy in infants and children. Pediatr. Clin. North Am. 1985;32:557–574. - PubMed
-
- Bracci L. Moschella F., et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin. Cancer Res. 2007;13(2 Pt 1):644–653. - PubMed
-
- Brentjens R.J. Santos E., et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin. Cancer Res. 2007;13(18 Pt 1):5426–5435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

