Pds5 promotes and protects cohesin acetylation
- PMID: 23878248
- PMCID: PMC3740900
- DOI: 10.1073/pnas.1306900110
Pds5 promotes and protects cohesin acetylation
Abstract
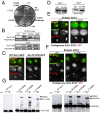
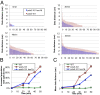
Cohesin's Smc1 and Smc3 subunits form V-shaped heterodimers, the nucleotide binding domains (NBDs) of which bind the C- and N-terminal domains, respectively, of the α-kleisin subunit, forming a large tripartite ring within in which sister DNAs are entrapped, and thereby held together (sister chromatid cohesion). During replication, establishment of stable cohesion is dependent on Eco1-mediated acetylation of Smc3's NBD, which is thought to prevent dissociation of α-kleisin from Smc3, thereby locking shut a "DNA exit gate." How Scc3 and Pds5, regulatory subunits bound to α-kleisin, regulate cohesion establishment and maintenance is poorly understood. We show here that by binding to α-kleisin adjacent to its Smc3 nucleotide binding N-terminal domain, Pds5 not only promotes cohesin's release from chromatin but also mediates de novo acetylation of Smc3 by Eco1 during S phase and subsequently prevents de-acetylation by the deacetylase Hos1/HDAC8. By first promoting cohesin's release from chromosomes and subsequently creating and guarding the chemical modification responsible for blocking release, Pds5 enables chromosomal cohesin to switch during S phase from a state of high turnover to one capable of tenaciously holding sister chromatids together for extended periods of time, a duality that has hitherto complicated analysis of this versatile cohesin subunit.
Keywords: cell; gene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

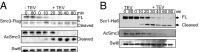

Similar articles
-
Building sister chromatid cohesion: smc3 acetylation counteracts an antiestablishment activity.Mol Cell. 2009 Mar 27;33(6):763-74. doi: 10.1016/j.molcel.2009.02.028. Mol Cell. 2009. PMID: 19328069
-
An Smc3 acetylation cycle is essential for establishment of sister chromatid cohesion.Mol Cell. 2010 Sep 10;39(5):689-99. doi: 10.1016/j.molcel.2010.08.008. Mol Cell. 2010. PMID: 20832721 Free PMC article.
-
Cohesin rings devoid of Scc3 and Pds5 maintain their stable association with the DNA.PLoS Genet. 2012;8(8):e1002856. doi: 10.1371/journal.pgen.1002856. Epub 2012 Aug 9. PLoS Genet. 2012. PMID: 22912589 Free PMC article.
-
Releasing the cohesin ring: A rigid scaffold model for opening the DNA exit gate by Pds5 and Wapl.Bioessays. 2017 Apr;39(4). doi: 10.1002/bies.201600207. Epub 2017 Feb 21. Bioessays. 2017. PMID: 28220956 Review.
-
The expanding phenotypes of cohesinopathies: one ring to rule them all!Cell Cycle. 2019 Nov;18(21):2828-2848. doi: 10.1080/15384101.2019.1658476. Epub 2019 Sep 13. Cell Cycle. 2019. PMID: 31516082 Free PMC article. Review.
Cited by
-
Transport of DNA within cohesin involves clamping on top of engaged heads by Scc2 and entrapment within the ring by Scc3.Elife. 2020 Sep 15;9:e59560. doi: 10.7554/eLife.59560. Elife. 2020. PMID: 32930661 Free PMC article.
-
Regulation of cohesin-mediated chromosome folding by PDS5 in mammals.EMBO Rep. 2022 Nov 7;23(11):e54853. doi: 10.15252/embr.202254853. Epub 2022 Sep 21. EMBO Rep. 2022. PMID: 36129789 Free PMC article.
-
PDS5A and PDS5B in Cohesin Function and Human Disease.Int J Mol Sci. 2021 May 30;22(11):5868. doi: 10.3390/ijms22115868. Int J Mol Sci. 2021. PMID: 34070827 Free PMC article. Review.
-
Genome folding through loop extrusion by SMC complexes.Nat Rev Mol Cell Biol. 2021 Jul;22(7):445-464. doi: 10.1038/s41580-021-00349-7. Epub 2021 Mar 25. Nat Rev Mol Cell Biol. 2021. PMID: 33767413 Review.
-
The nature of meiotic chromosome dynamics and recombination in budding yeast.J Microbiol. 2019 Apr;57(4):221-231. doi: 10.1007/s12275-019-8541-9. Epub 2019 Jan 22. J Microbiol. 2019. PMID: 30671743 Review.
References
-
- Haering CH, Löwe J, Hochwagen A, Nasmyth K. Molecular architecture of SMC proteins and the yeast cohesin complex. Mol Cell. 2002;9(4):773–788. - PubMed
-
- Haering CH, Farcas AM, Arumugam P, Metson J, Nasmyth K. The cohesin ring concatenates sister DNA molecules. Nature. 2008;454(7202):297–301. - PubMed
-
- Panizza S, Tanaka T, Hochwagen A, Eisenhaber F, Nasmyth K. Pds5 cooperates with cohesin in maintaining sister chromatid cohesion. Curr Biol. 2000;10(24):1557–1564. - PubMed
-
- Rankin S, Ayad NG, Kirschner MW. Sororin, a substrate of the anaphase-promoting complex, is required for sister chromatid cohesion in vertebrates. Mol Cell. 2005;18(2):185–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

