Production of α-galactosylceramide by a prominent member of the human gut microbiota
- PMID: 23874157
- PMCID: PMC3712910
- DOI: 10.1371/journal.pbio.1001610
Production of α-galactosylceramide by a prominent member of the human gut microbiota
Abstract
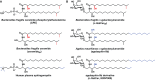
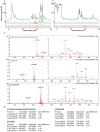
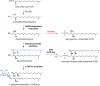
While the human gut microbiota are suspected to produce diffusible small molecules that modulate host signaling pathways, few of these molecules have been identified. Species of Bacteroides and their relatives, which often comprise >50% of the gut community, are unusual among bacteria in that their membrane is rich in sphingolipids, a class of signaling molecules that play a key role in inducing apoptosis and modulating the host immune response. Although known for more than three decades, the full repertoire of Bacteroides sphingolipids has not been defined. Here, we use a combination of genetics and chemistry to identify the sphingolipids produced by Bacteroides fragilis NCTC 9343. We constructed a deletion mutant of BF2461, a putative serine palmitoyltransferase whose yeast homolog catalyzes the committed step in sphingolipid biosynthesis. We show that the Δ2461 mutant is sphingolipid deficient, enabling us to purify and solve the structures of three alkaline-stable lipids present in the wild-type strain but absent from the mutant. The first compound was the known sphingolipid ceramide phosphorylethanolamine, and the second was its corresponding dihydroceramide base. Unexpectedly, the third compound was the glycosphingolipid α-galactosylceramide (α-GalCer(Bf)), which is structurally related to a sponge-derived sphingolipid (α-GalCer, KRN7000) that is the prototypical agonist of CD1d-restricted natural killer T (iNKT) cells. We demonstrate that α-GalCer(Bf) has similar immunological properties to KRN7000: it binds to CD1d and activates both mouse and human iNKT cells both in vitro and in vivo. Thus, our study reveals BF2461 as the first known member of the Bacteroides sphingolipid pathway, and it indicates that the committed steps of the Bacteroides and eukaryotic sphingolipid pathways are identical. Moreover, our data suggest that some Bacteroides sphingolipids might influence host immune homeostasis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Interaction between Bacteria and the Immune System for Cancer Immunotherapy: The α-GalCer Alliance.Int J Mol Sci. 2022 May 24;23(11):5896. doi: 10.3390/ijms23115896. Int J Mol Sci. 2022. PMID: 35682578 Free PMC article.
-
Bacterial immunogenic α-galactosylceramide identified in the murine large intestine: dependency on diet and inflammation.J Lipid Res. 2019 Nov;60(11):1892-1904. doi: 10.1194/jlr.RA119000236. Epub 2019 Sep 4. J Lipid Res. 2019. PMID: 31484693 Free PMC article.
-
Probiotic treatment with viable α-galactosylceramide-producing Bacteroides fragilis reduces diabetes incidence in female nonobese diabetic mice.J Diabetes. 2024 Aug;16(8):e13593. doi: 10.1111/1753-0407.13593. J Diabetes. 2024. PMID: 39136533 Free PMC article.
-
Tailored design of NKT-stimulatory glycolipids for polarization of immune responses.J Biomed Sci. 2017 Mar 23;24(1):22. doi: 10.1186/s12929-017-0325-0. J Biomed Sci. 2017. PMID: 28335781 Free PMC article. Review.
-
Invariant natural killer T cells: innate-like T cells with potent immunomodulatory activities.Tissue Antigens. 2009 Jun;73(6):535-45. doi: 10.1111/j.1399-0039.2009.01256.x. Epub 2009 Apr 8. Tissue Antigens. 2009. PMID: 19392798 Review.
Cited by
-
Glucosylceramide production maintains colon integrity in response to Bacteroides fragilis toxin-induced colon epithelial cell signaling.FASEB J. 2020 Dec;34(12):15922-15945. doi: 10.1096/fj.202001669R. Epub 2020 Oct 13. FASEB J. 2020. PMID: 33047400 Free PMC article.
-
Animals in a bacterial world: opportunities for chemical ecology.Nat Prod Rep. 2015 Jul;32(7):888-92. doi: 10.1039/c4np00141a. Nat Prod Rep. 2015. PMID: 25656944 Free PMC article. Review.
-
Invariant natural killer T cells: front line fighters in the war against pathogenic microbes.Immunogenetics. 2016 Aug;68(8):639-48. doi: 10.1007/s00251-016-0933-y. Epub 2016 Jul 1. Immunogenetics. 2016. PMID: 27368411 Free PMC article. Review.
-
The role of MHC class Ib-restricted T cells during infection.Immunogenetics. 2016 Aug;68(8):677-91. doi: 10.1007/s00251-016-0932-z. Epub 2016 Jul 1. Immunogenetics. 2016. PMID: 27368413 Free PMC article. Review.
-
DMS as an orthogonal separation to LC/ESI/MS/MS for quantifying isomeric cerebrosides in plasma and cerebrospinal fluid.J Lipid Res. 2019 Jan;60(1):200-211. doi: 10.1194/jlr.D089797. Epub 2018 Nov 9. J Lipid Res. 2019. PMID: 30413651 Free PMC article.
References
-
- Olsen I, Jantzen E (2001) Sphingolipids in bacteria and fungi. Anaerobe 7: 103–112.
-
- LaBach JP, White DC (1969) Identification of ceramide phosphorylethanolamine and ceramide phosphorylglycerol in the lipids of an anaerobic bacterium. J Lipid Res 10: 528–534. - PubMed
-
- White DC, Tucker AN, Sweeley CC (1969) Characterization of the iso-branched sphinganines from the ceramide phospholipids of Bacteroides melaninogenicus. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism 187: 527–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

