The redox biochemistry of protein sulfenylation and sulfinylation
- PMID: 23861405
- PMCID: PMC3772195
- DOI: 10.1074/jbc.R113.467738
The redox biochemistry of protein sulfenylation and sulfinylation
Abstract
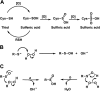
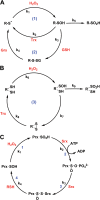
Controlled generation of reactive oxygen species orchestrates numerous physiological signaling events (Finkel, T. (2011) Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7-15). A major cellular target of reactive oxygen species is the thiol side chain (RSH) of Cys, which may assume a wide range of oxidation states (i.e. -2 to +4). Within this context, Cys sulfenic (Cys-SOH) and sulfinic (Cys-SO2H) acids have emerged as important mechanisms for regulation of protein function. Although this area has been under investigation for over a decade, the scope and biological role of sulfenic/sulfinic acid modifications have been recently expanded with the introduction of new tools for monitoring cysteine oxidation in vitro and directly in cells. This minireview discusses selected recent examples of protein sulfenylation and sulfinylation from the literature, highlighting the role of these post-translational modifications in cell signaling.
Keywords: Cysteine Oxidation; Hydrogen Peroxide; Post-translational Modification; Redox Regulation; Redox Signaling; Thiol.
Figures
Similar articles
-
The role of sulfenic acids in cellular redox signaling: Reconciling chemical kinetics and molecular detection strategies.Arch Biochem Biophys. 2017 Feb 15;616:40-46. doi: 10.1016/j.abb.2017.01.008. Epub 2017 Jan 23. Arch Biochem Biophys. 2017. PMID: 28126370 Free PMC article.
-
Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation.Biochemistry. 1999 Nov 23;38(47):15407-16. doi: 10.1021/bi992025k. Biochemistry. 1999. PMID: 10569923 Review.
-
A genetically encoded probe for cysteine sulfenic acid protein modification in vivo.Biochemistry. 2007 Dec 18;46(50):14725-32. doi: 10.1021/bi701625s. Epub 2007 Nov 20. Biochemistry. 2007. PMID: 18020457
-
RegB kinase activity is repressed by oxidative formation of cysteine sulfenic acid.J Biol Chem. 2013 Feb 15;288(7):4755-62. doi: 10.1074/jbc.M112.413492. Epub 2013 Jan 10. J Biol Chem. 2013. PMID: 23306201 Free PMC article.
-
Cysteine thiol sulfinic acid in plant stress signaling.Plant Cell Environ. 2024 Aug;47(8):2766-2779. doi: 10.1111/pce.14827. Epub 2024 Jan 22. Plant Cell Environ. 2024. PMID: 38251793 Review.
Cited by
-
Synthesis of benzopentathiepin analogs and their evaluation as inhibitors of the phosphatase STEP.Bioorg Med Chem Lett. 2015 Mar 1;25(5):1044-6. doi: 10.1016/j.bmcl.2015.01.020. Epub 2015 Jan 20. Bioorg Med Chem Lett. 2015. PMID: 25666825 Free PMC article.
-
Biological Functions of Hydrogen Sulfide in Plants.Int J Mol Sci. 2022 Dec 1;23(23):15107. doi: 10.3390/ijms232315107. Int J Mol Sci. 2022. PMID: 36499443 Free PMC article. Review.
-
The Plasma Membrane: A Platform for Intra- and Intercellular Redox Signaling.Antioxidants (Basel). 2018 Nov 20;7(11):168. doi: 10.3390/antiox7110168. Antioxidants (Basel). 2018. PMID: 30463362 Free PMC article. Review.
-
Molecular probes for cellular imaging of post-translational proteoforms.RSC Chem Biol. 2022 Jan 4;3(2):201-219. doi: 10.1039/d1cb00190f. eCollection 2022 Feb 9. RSC Chem Biol. 2022. PMID: 35360891 Free PMC article. Review.
-
RitR is an archetype for a novel family of redox sensors in the streptococci that has evolved from two-component response regulators and is required for pneumococcal colonization.PLoS Pathog. 2018 May 11;14(5):e1007052. doi: 10.1371/journal.ppat.1007052. eCollection 2018 May. PLoS Pathog. 2018. PMID: 29750817 Free PMC article.
References
-
- Ferrer-Sueta G., Manta B., Botti H., Radi R., Trujillo M., Denicola A. (2011) Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chem. Res. Toxicol. 24, 434–450 - PubMed
-
- Roos G., Foloppe N., Messens J. (2013) Understanding the pKa of redox cysteines: the key role of hydrogen bonding. Antioxid. Redox Signal. 18, 94–127 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

