Posttranslational modifications in type I collagen from different tissues extracted from wild type and prolyl 3-hydroxylase 1 null mice
- PMID: 23861401
- PMCID: PMC3750170
- DOI: 10.1074/jbc.M113.464156
Posttranslational modifications in type I collagen from different tissues extracted from wild type and prolyl 3-hydroxylase 1 null mice
Abstract
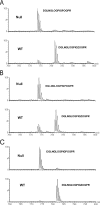
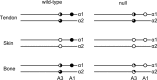
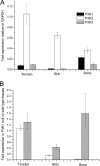
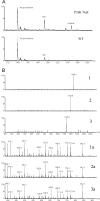
Type I collagen extracted from tendon, skin, and bone of wild type and prolyl 3-hydroxylase 1 (P3H1) null mice shows distinct patterns of 3-hydroxylation and glycosylation of hydroxylysine residues. The A1 site (Pro-986) in the α1-chain of type I collagen is almost completely 3-hydroxylated in every tissue of the wild type mice. In contrast, no 3-hydroxylation of this proline residue was found in P3H1 null mice. Partial 3-hydroxylation of the A3 site (Pro-707) was present in tendon and bone, but absent in skin in both α-chains of the wild type animals. Type I collagen extracted from bone of P3H1 null mice shows a large reduction in 3-hydroxylation of the A3 site in both α-chains, whereas type I collagen extracted from tendon of P3H1 null mice shows little difference as compared with wild type. These results demonstrate that the A1 site in type I collagen is exclusively 3-hydroxylated by P3H1, and presumably, this enzyme is required for the 3-hydroxylation of the A3 site of both α-chains in bone but not in tendon. The increase in glycosylation of hydroxylysine in P3H1 null mice in bone was found to be due to an increased occupancy of normally glycosylated sites. Despite the severe disorganization of collagen fibrils in adult tissues, the D-period of the fibrils is unchanged. Tendon fibrils of newborn P3H1 null mice are well organized with only a slight increase in diameter. The absence of 3-hydroxyproline and/or the increased glycosylation of hydroxylysine in type I collagen disturbs the lateral growth of the fibrils.
Keywords: Collagen; Hydroxylysine; Hydroxyproline; Post translational modification; Prolyl 3-hydroxylase 1; Protein synthesis; Tendon; glycosyl hydroxylysine.
Figures




Similar articles
-
Post-translationally abnormal collagens of prolyl 3-hydroxylase-2 null mice offer a pathobiological mechanism for the high myopia linked to human LEPREL1 mutations.J Biol Chem. 2015 Mar 27;290(13):8613-22. doi: 10.1074/jbc.M114.634915. Epub 2015 Feb 2. J Biol Chem. 2015. PMID: 25645914 Free PMC article.
-
Type I and type V procollagen triple helix uses different subsets of the molecular ensemble for lysine posttranslational modifications in the rER.J Biol Chem. 2021 Jan-Jun;296:100453. doi: 10.1016/j.jbc.2021.100453. Epub 2021 Feb 23. J Biol Chem. 2021. PMID: 33631195 Free PMC article.
-
Collagen prolyl 3-hydroxylation: a major role for a minor post-translational modification?Connect Tissue Res. 2013;54(4-5):245-51. doi: 10.3109/03008207.2013.800867. Epub 2013 Jun 21. Connect Tissue Res. 2013. PMID: 23772978 Free PMC article. Review.
-
Prolyl 3-hydroxylase 1 null mice display abnormalities in fibrillar collagen-rich tissues such as tendons, skin, and bones.J Biol Chem. 2010 May 28;285(22):17253-62. doi: 10.1074/jbc.M110.102228. Epub 2010 Apr 2. J Biol Chem. 2010. PMID: 20363744 Free PMC article.
-
Null mutations in LEPRE1 and CRTAP cause severe recessive osteogenesis imperfecta.Cell Tissue Res. 2010 Jan;339(1):59-70. doi: 10.1007/s00441-009-0872-0. Epub 2009 Oct 28. Cell Tissue Res. 2010. PMID: 19862557 Free PMC article. Review.
Cited by
-
Age-related type I collagen modifications reveal tissue-defining differences between ligament and tendon.Matrix Biol Plus. 2021 Jun 2;12:100070. doi: 10.1016/j.mbplus.2021.100070. eCollection 2021 Dec. Matrix Biol Plus. 2021. PMID: 34825162 Free PMC article.
-
Differential effects of collagen prolyl 3-hydroxylation on skeletal tissues.PLoS Genet. 2014 Jan;10(1):e1004121. doi: 10.1371/journal.pgen.1004121. Epub 2014 Jan 23. PLoS Genet. 2014. PMID: 24465224 Free PMC article.
-
Substitution of murine type I collagen A1 3-hydroxylation site alters matrix structure but does not recapitulate osteogenesis imperfecta bone dysplasia.Matrix Biol. 2020 Aug;90:20-39. doi: 10.1016/j.matbio.2020.02.003. Epub 2020 Feb 26. Matrix Biol. 2020. PMID: 32112888 Free PMC article.
-
A substrate preference for the rough endoplasmic reticulum resident protein FKBP22 during collagen biosynthesis.J Biol Chem. 2014 Jun 27;289(26):18189-201. doi: 10.1074/jbc.M114.561944. Epub 2014 May 12. J Biol Chem. 2014. PMID: 24821723 Free PMC article.
-
Comprehensive Characterization of Glycosylation and Hydroxylation of Basement Membrane Collagen IV by High-Resolution Mass Spectrometry.J Proteome Res. 2016 Jan 4;15(1):245-58. doi: 10.1021/acs.jproteome.5b00767. Epub 2015 Dec 9. J Proteome Res. 2016. PMID: 26593852 Free PMC article.
References
-
- Myllyharju J., Kivirikko K. I. (2004) Collagens, modifying enzymes and their mutations in humans, flies, and worms. Trends Genet. 20, 33–43 - PubMed
-
- Myllyharju J. (2003) Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 22, 15–24 - PubMed
-
- Pihlajaniemi T., Myllylä R., Kivirikko K. I. (1991) Prolyl 4-hydroxylase and its role in collagen synthesis. J. Hepatol. 13, Suppl, 3, S2–S7 - PubMed
-
- Ogle J. D., Arlinghaus R. B., Lgan M. A. (1962) 3-Hydroxyproline, a new amino acid of collagen. J. Biol. Chem. 237, 3667–3673 - PubMed
-
- Risteli J., Tryggvason K., Kivirikko K. I. (1977) Prolyl 3-hydroxylase: partial characterization of the enzyme from rat kidney cortex. Eur. J. Biochem. 73, 485–492 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

