Increased CDK1 activity determines the timing of kinetochore-microtubule attachments in meiosis I
- PMID: 23857768
- PMCID: PMC3718970
- DOI: 10.1083/jcb.201303019
Increased CDK1 activity determines the timing of kinetochore-microtubule attachments in meiosis I
Abstract
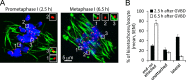
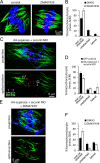
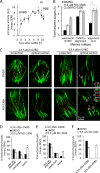
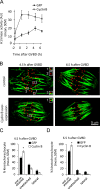
Chromosome segregation during cell division depends on stable attachment of kinetochores to spindle microtubules. Mitotic spindle formation and kinetochore-microtubule (K-MT) capture typically occur within minutes of nuclear envelope breakdown. In contrast, during meiosis I in mouse oocytes, formation of the acentrosomal bipolar spindle takes 3-4 h, and stabilization of K-MT attachments is delayed an additional 3-4 h. The mechanism responsible for this delay, which likely prevents stabilization of erroneous attachments during spindle formation, is unknown. Here we show that during meiosis I, attachments are regulated by CDK1 activity, which gradually increases through prometaphase and metaphase I. Partial reduction of CDK1 activity delayed formation of stable attachments, whereas a premature increase in CDK1 activity led to precocious formation of stable attachments and eventually lagging chromosomes at anaphase I. These results indicate that the slow increase in CDK1 activity in meiosis I acts as a timing mechanism to allow stable K-MT attachments only after bipolar spindle formation, thus preventing attachment errors.
Figures
Similar articles
-
Cdk1 negatively regulates the spindle localization of Prc1 in mouse oocytes.Genes Cells. 2020 Oct;25(10):685-694. doi: 10.1111/gtc.12803. Epub 2020 Sep 21. Genes Cells. 2020. PMID: 32865279
-
Gamma-tubulin is required for bipolar spindle assembly and for proper kinetochore microtubule attachments during prometaphase I in Drosophila oocytes.PLoS Genet. 2011 Aug;7(8):e1002209. doi: 10.1371/journal.pgen.1002209. Epub 2011 Aug 11. PLoS Genet. 2011. PMID: 21852952 Free PMC article.
-
Spatial Regulation of Kinetochore Microtubule Attachments by Destabilization at Spindle Poles in Meiosis I.Curr Biol. 2015 Jul 20;25(14):1835-41. doi: 10.1016/j.cub.2015.05.013. Epub 2015 Jul 9. Curr Biol. 2015. PMID: 26166779 Free PMC article.
-
Orchestration of the spindle assembly checkpoint by CDK1-cyclin B1.FEBS Lett. 2019 Oct;593(20):2889-2907. doi: 10.1002/1873-3468.13591. Epub 2019 Sep 13. FEBS Lett. 2019. PMID: 31469407 Review.
-
Detection and correction of merotelic kinetochore orientation by Aurora B and its partners.Cell Cycle. 2007 Jul 1;6(13):1558-64. doi: 10.4161/cc.6.13.4452. Epub 2007 May 18. Cell Cycle. 2007. PMID: 17603301 Review.
Cited by
-
Aneuploidy in Oocytes Is Prevented by Sustained CDK1 Activity through Degron Masking in Cyclin B1.Dev Cell. 2019 Mar 11;48(5):672-684.e5. doi: 10.1016/j.devcel.2019.01.008. Epub 2019 Feb 7. Dev Cell. 2019. PMID: 30745144 Free PMC article.
-
Cyclin B2 is required for progression through meiosis in mouse oocytes.Development. 2019 Apr 26;146(8):dev172734. doi: 10.1242/dev.172734. Development. 2019. PMID: 30952665 Free PMC article.
-
Condensin dysfunction is a reproductive isolating barrier in mice.Nature. 2023 Nov;623(7986):347-355. doi: 10.1038/s41586-023-06700-6. Epub 2023 Nov 1. Nature. 2023. PMID: 37914934 Free PMC article.
-
Mechanisms of kinetochore-microtubule attachment errors in mammalian oocytes.Dev Growth Differ. 2018 Jan;60(1):33-43. doi: 10.1111/dgd.12410. Epub 2018 Jan 10. Dev Growth Differ. 2018. PMID: 29318599 Free PMC article. Review.
-
Oocyte Maturation and Development.F1000Res. 2016 Mar 9;5:F1000 Faculty Rev-309. doi: 10.12688/f1000research.7892.1. eCollection 2016. F1000Res. 2016. PMID: 26998245 Free PMC article. Review.
References
-
- Aizawa H., Kamijo M., Ohba Y., Mori A., Okuhara K., Kawasaki H., Murofushi H., Suzuki K., Yasuda H. 1991. Microtubule destabilization by cdc2/H1 histone kinase: phosphorylation of a “pro-rich region” in the microtubule-binding domain of MAP-4. Biochem. Biophys. Res. Commun. 179:1620–1626 10.1016/0006-291X(91)91760-A - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

