Colorectal cancer intrinsic subtypes predict chemotherapy benefit, deficient mismatch repair and epithelial-to-mesenchymal transition
- PMID: 23852808
- PMCID: PMC4234005
- DOI: 10.1002/ijc.28387
Colorectal cancer intrinsic subtypes predict chemotherapy benefit, deficient mismatch repair and epithelial-to-mesenchymal transition
Abstract
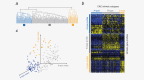
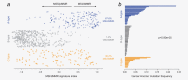
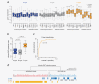
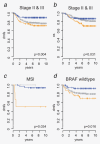
In most colorectal cancer (CRC) patients, outcome cannot be predicted because tumors with similar clinicopathological features can have differences in disease progression and treatment response. Therefore, a better understanding of the CRC biology is required to identify those patients who will benefit from chemotherapy and to find a more tailored therapy plan for other patients. Based on unsupervised classification of whole genome data from 188 stages I-IV CRC patients, a molecular classification was developed that consist of at least three major intrinsic subtypes (A-, B- and C-type). The subtypes were validated in 543 stages II and III patients and were associated with prognosis and benefit from chemotherapy. The heterogeneity of the intrinsic subtypes is largely based on three biological hallmarks of the tumor: epithelial-to-mesenchymal transition, deficiency in mismatch repair genes that result in high mutation frequency associated with microsatellite instability and cellular proliferation. A-type tumors, observed in 22% of the patients, have the best prognosis, have frequent BRAF mutations and a deficient DNA mismatch repair system. C-type patients (16%) have the worst outcome, a mesenchymal gene expression phenotype and show no benefit from adjuvant chemotherapy treatment. Both A-type and B-type tumors have a more proliferative and epithelial phenotype and B-types benefit from adjuvant chemotherapy. B-type tumors (62%) show a low overall mutation frequency consistent with the absence of DNA mismatch repair deficiency. Classification based on molecular subtypes made it possible to expand and improve CRC classification beyond standard molecular and immunohistochemical assessment and might help in the future to guide treatment in CRC patients.
Keywords: EMT; chemotherapy benefit; colorectal cancer; mismatch repair; molecular subtypes.
© 2013 UICC.
Figures

Similar articles
-
The Impact of Mismatch Repair Status in Colorectal Cancer on the Decision to Treat With Adjuvant Chemotherapy: An Australian Population-Based Multicenter Study.Oncologist. 2016 May;21(5):618-25. doi: 10.1634/theoncologist.2015-0530. Epub 2016 Mar 23. Oncologist. 2016. PMID: 27009937 Free PMC article.
-
Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer.Gut. 2006 Jun;55(6):848-55. doi: 10.1136/gut.2005.073015. Epub 2005 Nov 18. Gut. 2006. PMID: 16299036 Free PMC article.
-
Role of Deficient Mismatch Repair in the Personalized Management of Colorectal Cancer.Int J Environ Res Public Health. 2016 Sep 8;13(9):892. doi: 10.3390/ijerph13090892. Int J Environ Res Public Health. 2016. PMID: 27618077 Free PMC article. Review.
-
[Molecular biology in clinical cancer research: the example of digestive cancers].Rev Epidemiol Sante Publique. 2005 Jun;53(3):267-82. doi: 10.1016/s0398-7620(05)84604-8. Rev Epidemiol Sante Publique. 2005. PMID: 16227914 Review. French.
-
An immune, stroma, and epithelial-mesenchymal transition-related signature for predicting recurrence and chemotherapy benefit in stage II-III colorectal cancer.Cancer Med. 2023 Apr;12(7):8924-8936. doi: 10.1002/cam4.5534. Epub 2023 Jan 11. Cancer Med. 2023. PMID: 36629124 Free PMC article.
Cited by
-
Microsatellite Instability in Colorectal Cancer Liquid Biopsy-Current Updates on Its Potential in Non-Invasive Detection, Prognosis and as a Predictive Marker.Diagnostics (Basel). 2021 Mar 18;11(3):544. doi: 10.3390/diagnostics11030544. Diagnostics (Basel). 2021. PMID: 33803882 Free PMC article. Review.
-
Evaluation of the Use of Cell Lines in Studies of Selenium-Dependent Glutathione Peroxidase 2 (GPX2) Involvement in Colorectal Cancer.Diseases. 2024 Sep 10;12(9):207. doi: 10.3390/diseases12090207. Diseases. 2024. PMID: 39329876 Free PMC article.
-
RIP140 regulates POLK gene expression and the response to alkylating drugs in colon cancer cells.Cancer Drug Resist. 2022 May 7;5(2):401-414. doi: 10.20517/cdr.2021.133. eCollection 2022. Cancer Drug Resist. 2022. PMID: 35800380 Free PMC article.
-
Single Cell Transcriptome in Colorectal Cancer-Current Updates on Its Application in Metastasis, Chemoresistance and the Roles of Circulating Tumor Cells.Front Pharmacol. 2020 Feb 27;11:135. doi: 10.3389/fphar.2020.00135. eCollection 2020. Front Pharmacol. 2020. PMID: 32174835 Free PMC article. Review.
-
A panel of DNA methylation markers for the classification of consensus molecular subtypes 2 and 3 in patients with colorectal cancer.Mol Oncol. 2021 Dec;15(12):3348-3362. doi: 10.1002/1878-0261.13098. Epub 2021 Sep 30. Mol Oncol. 2021. PMID: 34510716 Free PMC article.
References
-
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. - PubMed
-
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. - PubMed
-
- Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

