Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture
- PMID: 23842490
- PMCID: PMC3739998
- DOI: 10.1038/nature12298
Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture
Abstract
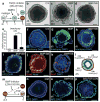
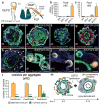
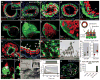
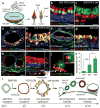
The inner ear contains sensory epithelia that detect head movements, gravity and sound. It is unclear how to develop these sensory epithelia from pluripotent stem cells, a process that will be critical for modelling inner ear disorders or developing cell-based therapies for profound hearing loss and balance disorders. So far, attempts to derive inner ear mechanosensitive hair cells and sensory neurons have resulted in inefficient or incomplete phenotypic conversion of stem cells into inner-ear-like cells. A key insight lacking from these previous studies is the importance of the non-neural and preplacodal ectoderm, two critical precursors during inner ear development. Here we report the stepwise differentiation of inner ear sensory epithelia from mouse embryonic stem cells (ESCs) in three-dimensional culture. We show that by recapitulating in vivo development with precise temporal control of signalling pathways, ESC aggregates transform sequentially into non-neural, preplacodal and otic-placode-like epithelia. Notably, in a self-organized process that mimics normal development, vesicles containing prosensory cells emerge from the presumptive otic placodes and give rise to hair cells bearing stereocilia bundles and a kinocilium. Moreover, these stem-cell-derived hair cells exhibit functional properties of native mechanosensitive hair cells and form specialized synapses with sensory neurons that have also arisen from ESCs in the culture. Finally, we demonstrate how these vesicles are structurally and biochemically comparable to developing vestibular end organs. Our data thus establish a new in vitro model of inner ear differentiation that can be used to gain deeper insight into inner ear development and disorder.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Directed Differentiation of Mouse Embryonic Stem Cells Into Inner Ear Sensory Epithelia in 3D Culture.Methods Mol Biol. 2017;1597:67-83. doi: 10.1007/978-1-4939-6949-4_6. Methods Mol Biol. 2017. PMID: 28361311 Free PMC article.
-
Generation of inner ear organoids from human pluripotent stem cells.Methods Cell Biol. 2020;159:303-321. doi: 10.1016/bs.mcb.2020.02.006. Epub 2020 Mar 11. Methods Cell Biol. 2020. PMID: 32586448
-
Directed Differentiation of Human Pluripotent Stem Cells into Inner Ear Organoids.Methods Mol Biol. 2022;2520:135-150. doi: 10.1007/7651_2021_448. Methods Mol Biol. 2022. PMID: 34724191
-
Building inner ears: recent advances and future challenges for in vitro organoid systems.Cell Death Differ. 2021 Jan;28(1):24-34. doi: 10.1038/s41418-020-00678-8. Epub 2020 Dec 14. Cell Death Differ. 2021. PMID: 33318601 Free PMC article. Review.
-
Development of the inner ear.Curr Opin Genet Dev. 2015 Jun;32:112-8. doi: 10.1016/j.gde.2015.02.006. Epub 2015 Mar 19. Curr Opin Genet Dev. 2015. PMID: 25796080 Review.
Cited by
-
Modelling inner ear development and disease using pluripotent stem cells - a pathway to new therapeutic strategies.Dis Model Mech. 2022 Nov 1;15(11):dmm049593. doi: 10.1242/dmm.049593. Epub 2022 Nov 4. Dis Model Mech. 2022. PMID: 36331565 Free PMC article. Review.
-
Stem Cell-Based Therapeutic Approaches to Restore Sensorineural Hearing Loss in Mammals.Neural Plast. 2020 Aug 1;2020:8829660. doi: 10.1155/2020/8829660. eCollection 2020. Neural Plast. 2020. PMID: 32802037 Free PMC article. Review.
-
Stem cell-based approaches: Possible route to hearing restoration?World J Stem Cells. 2020 Jun 26;12(6):422-437. doi: 10.4252/wjsc.v12.i6.422. World J Stem Cells. 2020. PMID: 32742560 Free PMC article. Review.
-
Biohybrid cochlear implants in human neurosensory restoration.Stem Cell Res Ther. 2016 Oct 7;7(1):148. doi: 10.1186/s13287-016-0408-y. Stem Cell Res Ther. 2016. PMID: 27717379 Free PMC article.
-
A Novel in vitro Model Delineating Hair Cell Regeneration and Neural Reinnervation in Adult Mouse Cochlea.Front Mol Neurosci. 2022 Jan 10;14:757831. doi: 10.3389/fnmol.2021.757831. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35082601 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

