The N-terminal domains of Vps3 and Vps8 are critical for localization and function of the CORVET tethering complex on endosomes
- PMID: 23840658
- PMCID: PMC3688683
- DOI: 10.1371/journal.pone.0067307
The N-terminal domains of Vps3 and Vps8 are critical for localization and function of the CORVET tethering complex on endosomes
Abstract
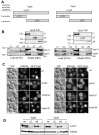
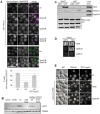
Endosomal biogenesis depends on multiple fusion and fission events. For fusion, the heterohexameric CORVET complex as an effector of the endosomal Rab5/Vps21 GTPase has a central function in the initial tethering event. Here, we show that the CORVET-specific Vps3 and Vps8 subunits, which interact with Rab5/Vps21, require their N-terminal domains for localization and function. Surprisingly, CORVET may lack either one of the two N-terminal domains, but not both, to promote protein sorting via the endosome. The dually truncated complex mislocalizes to the cytosol and is impaired in endocytic protein sorting, but not in assembly. Furthermore, the endosomal localization can be rescued by overexpression of Vps21 or one of the truncated CORVET subunits, even though CORVET assembly is not impaired by loss of the N-terminal domains or in strains lacking all endosomal Rab5s and Ypt7. We thus conclude that CORVET requires only its C-terminal domains for assembly and has beyond its putative β-propeller domains additional binding sites for endosomes, which could be important to bind Vps21 and other endosome-specific factors for efficient endosome tethering.
Conflict of interest statement
Figures


Similar articles
-
The CORVET subunit Vps8 cooperates with the Rab5 homolog Vps21 to induce clustering of late endosomal compartments.Mol Biol Cell. 2009 Dec;20(24):5276-89. doi: 10.1091/mbc.e09-06-0521. Mol Biol Cell. 2009. PMID: 19828734 Free PMC article.
-
Subunit organization and Rab interactions of Vps-C protein complexes that control endolysosomal membrane traffic.Mol Biol Cell. 2011 Apr 15;22(8):1353-63. doi: 10.1091/mbc.E10-03-0260. Epub 2011 Feb 16. Mol Biol Cell. 2011. PMID: 21325627 Free PMC article.
-
Mapping of Vps21 and HOPS binding sites in Vps8 and effect of binding site mutants on endocytic trafficking.Eukaryot Cell. 2010 Apr;9(4):602-10. doi: 10.1128/EC.00286-09. Epub 2010 Feb 19. Eukaryot Cell. 2010. PMID: 20173035 Free PMC article.
-
CORVET and HOPS tethering complexes - coordinators of endosome and lysosome fusion.J Cell Sci. 2013 Mar 15;126(Pt 6):1307-16. doi: 10.1242/jcs.107805. J Cell Sci. 2013. PMID: 23645161 Review.
-
Membrane dynamics and fusion at late endosomes and vacuoles--Rab regulation, multisubunit tethering complexes and SNAREs.Eur J Cell Biol. 2011 Sep;90(9):779-85. doi: 10.1016/j.ejcb.2011.04.007. Epub 2011 Jun 16. Eur J Cell Biol. 2011. PMID: 21683469 Review.
Cited by
-
Subunit organisation of in vitro reconstituted HOPS and CORVET multisubunit membrane tethering complexes.PLoS One. 2013 Dec 2;8(12):e81534. doi: 10.1371/journal.pone.0081534. eCollection 2013. PLoS One. 2013. PMID: 24312556 Free PMC article.
-
Structural identification of the Vps18 β-propeller reveals a critical role in the HOPS complex stability and function.J Biol Chem. 2014 Nov 28;289(48):33503-12. doi: 10.1074/jbc.M114.602714. Epub 2014 Oct 16. J Biol Chem. 2014. PMID: 25324549 Free PMC article.
-
Molecular etiology of arthrogryposis in multiple families of mostly Turkish origin.J Clin Invest. 2016 Feb;126(2):762-78. doi: 10.1172/JCI84457. Epub 2016 Jan 11. J Clin Invest. 2016. PMID: 26752647 Free PMC article. Clinical Trial.
-
Tracking of the dynamic localization of the Rab-specific HOPS subunits reveal their distinct interaction with Ypt7 and vacuoles.Cell Logist. 2014 May 12;4:e29191. doi: 10.4161/cl.29191. eCollection 2014. Cell Logist. 2014. PMID: 25210650 Free PMC article.
-
Overexpression of the CORVET complex alleviates the fungicidal effects of fludioxonil on the yeast Saccharomyces cerevisiae expressing hybrid histidine kinase 3.J Biol Chem. 2019 Jan 11;294(2):461-475. doi: 10.1074/jbc.RA118.004736. Epub 2018 Nov 16. J Biol Chem. 2019. PMID: 30446623 Free PMC article.
References
-
- Huotari J, Helenius A (2011) Endosome maturation. The EMBO Journal 30: 3481–3500 doi:10.1038/emboj.2011.286 - DOI - PMC - PubMed
-
- Epp N, Rethmeier R, Krämer L, Ungermann C (2011) Membrane dynamics and fusion at late endosomes and vacuoles–Rab regulation, multisubunit tethering complexes and SNAREs. Eur J Cell Biol 90: 779–785 doi:10.1016/j.ejcb.2011.04.007 - DOI - PubMed
-
- Hutagalung AH, Novick PJ (2011) Role of Rab GTPases in Membrane Traffic and Cell Physiology. Physiological Reviews 91: 119–149 doi:10.1152/physrev.00059.2009 - DOI - PMC - PubMed
-
- Barr F, Lambright DG (2010) Rab GEFs and GAPs. Curr Opin Cell Biol 22: 461–470 doi:10.1016/j.ceb.2010.04.007 - DOI - PMC - PubMed
-
- Schoebel S, Oesterlin LK, Blankenfeldt W, Goody RS, Itzen A (2009) RabGDI displacement by DrrA from Legionella is a consequence of its guanine nucleotide exchange activity. Mol Cell 36: 1060–1072 doi:10.1016/j.molcel.2009.11.014 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

