Receptor-mediated endocytosis and brain delivery of therapeutic biologics
- PMID: 23840214
- PMCID: PMC3693099
- DOI: 10.1155/2013/703545
Receptor-mediated endocytosis and brain delivery of therapeutic biologics
Abstract
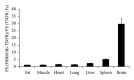
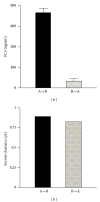
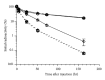
Transport of macromolecules across the blood-brain-barrier (BBB) requires both specific and nonspecific interactions between macromolecules and proteins/receptors expressed on the luminal and/or the abluminal surfaces of the brain capillary endothelial cells. Endocytosis and transcytosis play important roles in the distribution of macromolecules. Due to the tight junction of BBB, brain delivery of traditional therapeutic proteins with large molecular weight is generally not possible. There are multiple pathways through which macromolecules can be taken up into cells through both specific and nonspecific interactions with proteins/receptors on the cell surface. This review is focused on the current knowledge of receptor-mediated endocytosis/transcytosis and brain delivery using the Angiopep-2-conjugated system and the molecular Trojan horses. In addition, the role of neonatal Fc receptor (FcRn) in regulating the efflux of Immunoglobulin G (IgG) from brain to blood, and approaches to improve the pharmacokinetics of therapeutic biologics by generating Fc fusion proteins, and increasing the pH dependent binding affinity between Fc and FcRn, are discussed.
Figures
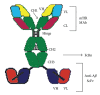
Similar articles
-
Adsorptive-Mediated Endocytosis of Sulfo-Cy5-Labeled IgG Causes Aberrant IgG Processing by Brain Endothelial-Like Cells.Mol Pharm. 2020 Nov 2;17(11):4280-4285. doi: 10.1021/acs.molpharmaceut.0c00712. Epub 2020 Oct 9. Mol Pharm. 2020. PMID: 32986439
-
Modifying antibody-FcRn interactions to increase the transport of antibodies through the blood-brain barrier.MAbs. 2023 Jan-Dec;15(1):2229098. doi: 10.1080/19420862.2023.2229098. MAbs. 2023. PMID: 37381177 Free PMC article.
-
In vitro and in vivo methods for assessing FcRn-mediated reverse transcytosis across the blood-brain barrier.Methods Mol Biol. 2011;763:383-401. doi: 10.1007/978-1-61779-191-8_26. Methods Mol Biol. 2011. PMID: 21874466
-
Receptor-mediated transcytosis of macromolecules across the blood-brain barrier.Expert Opin Drug Deliv. 2023 Jul-Dec;20(12):1699-1711. doi: 10.1080/17425247.2023.2255138. Epub 2023 Sep 15. Expert Opin Drug Deliv. 2023. PMID: 37658673 Review.
-
Blood-brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody.Expert Opin Drug Deliv. 2015 Feb;12(2):207-22. doi: 10.1517/17425247.2014.952627. Epub 2014 Aug 20. Expert Opin Drug Deliv. 2015. PMID: 25138991 Review.
Cited by
-
Drug delivery strategies to enhance the permeability of the blood-brain barrier for treatment of glioma.Drug Des Devel Ther. 2015 Apr 9;9:2089-100. doi: 10.2147/DDDT.S79592. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25926719 Free PMC article. Review.
-
Translational Approaches for Brain Delivery of Biologics via Cerebrospinal Fluid.Clin Pharmacol Ther. 2022 Apr;111(4):826-834. doi: 10.1002/cpt.2531. Epub 2022 Feb 23. Clin Pharmacol Ther. 2022. PMID: 35064573 Free PMC article. Review.
-
Designing and Characterization of a Novel Delivery System for Improved Cellular Uptake by Brain Using Dendronised Apo-E-Derived Peptide.Front Bioeng Biotechnol. 2019 Mar 26;7:49. doi: 10.3389/fbioe.2019.00049. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 30972332 Free PMC article.
-
Delivery of Therapeutic Agents to the Central Nervous System and the Promise of Extracellular Vesicles.Pharmaceutics. 2021 Apr 3;13(4):492. doi: 10.3390/pharmaceutics13040492. Pharmaceutics. 2021. PMID: 33916841 Free PMC article. Review.
-
Targeting therapeutics across the blood brain barrier (BBB), prerequisite towards thrombolytic therapy for cerebrovascular disorders-an overview and advancements.AAPS PharmSciTech. 2015 Apr;16(2):223-33. doi: 10.1208/s12249-015-0287-z. Epub 2015 Jan 23. AAPS PharmSciTech. 2015. PMID: 25613561 Free PMC article. Review.
References
-
- Blumling JP, III, Silva GA. Targeting the brain: advances in drug delivery. Current Pharmaceutical Biotechnology. 2012;13:2417–2426. - PubMed
-
- Gabathuler R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiology of Disease. 2010;37(1):48–57. - PubMed
-
- Demeule M, Currie J, Bertrand Y, et al. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2. Journal of Neurochemistry. 2008;106(4):1534–1544. - PubMed
-
- Demeule M, Regina A, Ché C, et al. Identification and design of peptides as a new drug delivery system for the brain. Journal of Pharmacology and Experimental Therapeutics. 2008;324(3):1064–1072. - PubMed
-
- Guo J, Gao X, Su L, et al. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32(31):8010–8020. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

