Human innate immunity to Toxoplasma gondii is mediated by host caspase-1 and ASC and parasite GRA15
- PMID: 23839215
- PMCID: PMC3705447
- DOI: 10.1128/mBio.00255-13
Human innate immunity to Toxoplasma gondii is mediated by host caspase-1 and ASC and parasite GRA15
Abstract
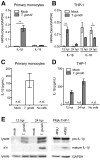
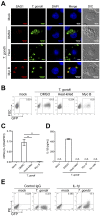
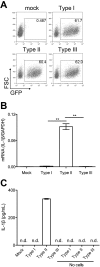
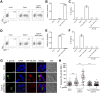
Interleukin-1β (IL-1β) functions as a key regulator of inflammation and innate immunity. The protozoan parasite Toxoplasma gondii actively infects human blood monocytes and induces the production of IL-1β; however, the host and parasite factors that mediate IL-1β production during T. gondii infection are poorly understood. We report that T. gondii induces IL-1β transcript, processing/cleavage, and release from infected primary human monocytes and THP-1 cells. Treating monocytes with the caspase-1 inhibitor Ac-YVAD-CMK reduced IL-1β release, suggesting a role for the inflammasome in T. gondii-induced IL-1β production. This was confirmed by performing short hairpin RNA (shRNA) knockdown of caspase-1 and of the inflammasome adaptor protein ASC. IL-1β induction required active parasite invasion of monocytes, since heat-killed or mycalolide B-treated parasites did not induce IL-1β. Among the type I, II, and III strains of T. gondii, the type II strain induced substantially more IL-1β mRNA and protein release than did the type I and III strains. Since IL-1β transcript is known to be induced downstream of NF-κB signaling, we investigated a role for the GRA15 protein, which induces sustained NF-κB signaling in a parasite strain-specific manner. By infecting human monocytes with a GRA15-knockout type II strain and a type I strain stably expressing type II GRA15, we determined that GRA15 is responsible for IL-1β induction during T. gondii infection of human monocytes. This research defines a pathway driving human innate immunity by describing a role for the classical inflammasome components caspase-1 and ASC and the parasite GRA15 protein in T. gondii-induced IL-1β production.
Importance: Monocytes are immune cells that protect against infection by increasing inflammation and antimicrobial activities in the body. Upon infection with the parasitic pathogen Toxoplasma gondii, human monocytes release interleukin-1β (IL-1β), a "master regulator" of inflammation, which amplifies immune responses. Although inflammatory responses are critical for host defense against infection, excessive inflammation can result in tissue damage and pathology. This delicate balance underscores the importance of understanding the mechanisms that regulate IL-1β during infection. We have investigated the molecular pathway by which T. gondii induces the synthesis and release of IL-1β in human monocytes. We found that specific proteins in the parasite and the host cell coordinate to induce IL-1β production. This research is significant because it contributes to a greater understanding of human innate immunity to infection and IL-1β regulation, thereby enhancing our potential to modulate inflammation in the body.
Figures


Similar articles
-
Toxoplasma gondii activates a Syk-CARD9-NF-κB signaling axis and gasdermin D-independent release of IL-1β during infection of primary human monocytes.PLoS Pathog. 2019 Aug 26;15(8):e1007923. doi: 10.1371/journal.ppat.1007923. eCollection 2019 Aug. PLoS Pathog. 2019. PMID: 31449558 Free PMC article.
-
Evasion of Human Neutrophil-Mediated Host Defense during Toxoplasma gondii Infection.mBio. 2018 Feb 13;9(1):e02027-17. doi: 10.1128/mBio.02027-17. mBio. 2018. PMID: 29440572 Free PMC article.
-
Inducible Nitric Oxide Synthase Is a Key Host Factor for Toxoplasma GRA15-Dependent Disruption of the Gamma Interferon-Induced Antiparasitic Human Response.mBio. 2018 Oct 9;9(5):e01738-18. doi: 10.1128/mBio.01738-18. mBio. 2018. PMID: 30301855 Free PMC article.
-
From cells to signaling cascades: manipulation of innate immunity by Toxoplasma gondii.FEMS Immunol Med Microbiol. 2003 Dec 5;39(3):193-203. doi: 10.1016/S0928-8244(03)00279-7. FEMS Immunol Med Microbiol. 2003. PMID: 14642303 Review.
-
Cell intrinsic roles of apoptosis-associated speck-like protein in regulating innate and adaptive immune responses.ScientificWorldJournal. 2011;11:2418-23. doi: 10.1100/2011/429192. Epub 2011 Dec 8. ScientificWorldJournal. 2011. PMID: 22194672 Free PMC article. Review.
Cited by
-
Caspase-11 Modulates Inflammation and Attenuates Toxoplasma gondii Pathogenesis.Mediators Inflamm. 2016;2016:9848263. doi: 10.1155/2016/9848263. Epub 2016 Jun 9. Mediators Inflamm. 2016. PMID: 27378827 Free PMC article.
-
Mechanisms of Human Innate Immune Evasion by Toxoplasma gondii.Front Cell Infect Microbiol. 2019 Apr 16;9:103. doi: 10.3389/fcimb.2019.00103. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31041194 Free PMC article. Review.
-
NLRP1 is an inflammasome sensor for Toxoplasma gondii.Infect Immun. 2014 Jan;82(1):460-8. doi: 10.1128/IAI.01170-13. Epub 2013 Nov 11. Infect Immun. 2014. PMID: 24218483 Free PMC article.
-
Role of interleukin 1β and interleukin 10 variants on ocular toxoplasmosis in Brazilian individuals.Front Ophthalmol (Lausanne). 2023 Jun 29;3:1183167. doi: 10.3389/fopht.2023.1183167. eCollection 2023. Front Ophthalmol (Lausanne). 2023. PMID: 38983057 Free PMC article.
-
Toxoplasma gondii activates a Syk-CARD9-NF-κB signaling axis and gasdermin D-independent release of IL-1β during infection of primary human monocytes.PLoS Pathog. 2019 Aug 26;15(8):e1007923. doi: 10.1371/journal.ppat.1007923. eCollection 2019 Aug. PLoS Pathog. 2019. PMID: 31449558 Free PMC article.
References
-
- Dubey JP. 2008. The history of Toxoplasma gondii—the first 100 years. J. Eukaryot. Microbiol. 55:467–475 - PubMed
-
- Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976 - PubMed
-
- Luft BJ, Remington JS. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15:211–222 - PubMed
-
- Jones JL, Lopez A, Wilson M, Schulkin J, Gibbs R. 2001. Congenital toxoplasmosis: a review. Obstet. Gynecol. Surv. 56:296–305 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
