Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility
- PMID: 23838163
- PMCID: PMC3798034
- DOI: 10.1161/CIRCULATIONAHA.113.002714
Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility
Abstract
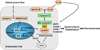
Background: The molecular basis for the focal nature of atherosclerotic lesions is poorly understood. Here, we explored whether disturbed flow patterns activate an innate immune response to form the NLRP3 inflammasome scaffold in vascular endothelial cells via sterol regulatory element binding protein 2 (SREBP2).
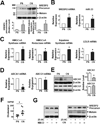
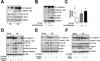
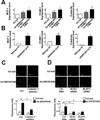
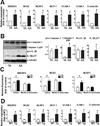
Methods and results: Oscillatory flow activates SREBP2 and induces NLRP3 inflammasome in endothelial cells. The underlying mechanisms involve SREBP2 transactivating NADPH oxidase 2 and NLRP3. Consistently, SREBP2, NADPH oxidase 2, and NLRP3 levels were elevated in atheroprone areas of mouse aortas, suggesting that the SREBP2-activated NLRP3 inflammasome causes functionally disturbed endothelium with increased inflammation. Mimicking the effect of atheroprone flow, endothelial cell-specific overexpression of the activated form of SREBP2 synergized with hyperlipidemia to increase atherosclerosis in the atheroresistant areas of mouse aortas.
Conclusions: Atheroprone flow induces NLRP3 inflammasome in endothelium through SREBP2 activation. This increased innate immunity in endothelium synergizes with hyperlipidemia to cause topographical distribution of atherosclerotic lesions.
Keywords: NLRP3 protein, human; atherosclerosis; endothelial cell; shear stress; sterol regulatory element binding proteins.
Conflict of interest statement
Figures



Comment in
-
Atheroprone flow activation of the sterol regulatory element binding protein 2 and nod-like receptor protein 3 inflammasome mediates focal atherosclerosis.Circulation. 2013 Aug 6;128(6):579-82. doi: 10.1161/CIRCULATIONAHA.113.004390. Epub 2013 Jul 9. Circulation. 2013. PMID: 23838164 Free PMC article.
Similar articles
-
Oxidative stress activates endothelial innate immunity via sterol regulatory element binding protein 2 (SREBP2) transactivation of microRNA-92a.Circulation. 2015 Mar 3;131(9):805-14. doi: 10.1161/CIRCULATIONAHA.114.013675. Epub 2014 Dec 30. Circulation. 2015. PMID: 25550450 Free PMC article.
-
TIFA as a crucial mediator for NLRP3 inflammasome.Proc Natl Acad Sci U S A. 2016 Dec 27;113(52):15078-15083. doi: 10.1073/pnas.1618773114. Epub 2016 Dec 13. Proc Natl Acad Sci U S A. 2016. PMID: 27965388 Free PMC article.
-
Atheroprone flow activation of the sterol regulatory element binding protein 2 and nod-like receptor protein 3 inflammasome mediates focal atherosclerosis.Circulation. 2013 Aug 6;128(6):579-82. doi: 10.1161/CIRCULATIONAHA.113.004390. Epub 2013 Jul 9. Circulation. 2013. PMID: 23838164 Free PMC article.
-
Endothelial dysfunction: the role of sterol regulatory element-binding protein-induced NOD-like receptor family pyrin domain-containing protein 3 inflammasome in atherosclerosis.Curr Opin Lipidol. 2014 Oct;25(5):339-49. doi: 10.1097/MOL.0000000000000107. Curr Opin Lipidol. 2014. PMID: 25188917 Free PMC article. Review.
-
NLRP3 inflammasome pathways in atherosclerosis.Atherosclerosis. 2017 Dec;267:127-138. doi: 10.1016/j.atherosclerosis.2017.10.027. Epub 2017 Oct 22. Atherosclerosis. 2017. PMID: 29126031 Review.
Cited by
-
Mechanisms that regulate macrophage burden in atherosclerosis.Circ Res. 2014 May 23;114(11):1757-71. doi: 10.1161/CIRCRESAHA.114.301174. Circ Res. 2014. PMID: 24855200 Free PMC article. Review.
-
Anti-inflammatory therapies for cardiovascular disease.Eur Heart J. 2014 Jul 14;35(27):1782-91. doi: 10.1093/eurheartj/ehu203. Epub 2014 May 26. Eur Heart J. 2014. PMID: 24864079 Free PMC article. Review.
-
Bibliometric evaluation of publications on inflammasomes in atherosclerosis from 2002 to 2022.Front Cardiovasc Med. 2023 Apr 12;10:1067226. doi: 10.3389/fcvm.2023.1067226. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37123477 Free PMC article.
-
Endothelial fluid shear stress sensing in vascular health and disease.J Clin Invest. 2016 Mar 1;126(3):821-8. doi: 10.1172/JCI83083. Epub 2016 Mar 1. J Clin Invest. 2016. PMID: 26928035 Free PMC article. Review.
-
Flow signaling and atherosclerosis.Cell Mol Life Sci. 2017 May;74(10):1835-1858. doi: 10.1007/s00018-016-2442-4. Epub 2016 Dec 30. Cell Mol Life Sci. 2017. PMID: 28039525 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

