Cyclin D1 affects epithelial-mesenchymal transition in epithelial ovarian cancer stem cell-like cells
- PMID: 23836980
- PMCID: PMC3699300
- DOI: 10.2147/OTT.S44177
Cyclin D1 affects epithelial-mesenchymal transition in epithelial ovarian cancer stem cell-like cells
Abstract
Background: The association of cancer stem cells with epithelial-mesenchymal transition (EMT) is receiving attention. We found in our previous study that EMT existed from CD24- phenotype cells to their differentiated cells. It was shown that cyclin D1 functioned in sustaining self-renewal independent of CDK4/CDK6 activation, but its effect on the EMT mechanism in ovarian cancer stem cells is unclear.
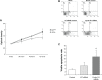
Methods: The anchorage-independent spheroids from ovarian adenocarcinoma cell line 3AO were formed in a serum-free medium. CD24- and CD24+ cells were isolated by fluorescence-activated cell sorting. Cell morphology, viability, apoptosis, and migratory ability were observed. Stem-related molecule Bmi-1, Oct-4 and EMT-related marker E-cadherin, and vimentin expressions were analyzed. Cyclin D1 expression in CD24- phenotype enriched spheroids was knocked down with small interfering RNA, and its effects on cell proliferation, apoptosis, migration ability, and EMT-related phenotype after transfection were observed.
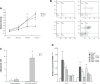
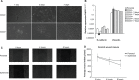
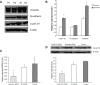
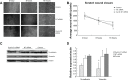
Results: In our study, CD24- cells presented stronger proliferative, anti-apoptosis capacity, and migratory ability, than CD24+ cells or parental cells. CD24- cells grew with a scattered spindle-shape within 3 days of culture and transformed into a cobblestone-like shape, identical to CD24+ cells or parental cells at 7 days of culture. CD24- cells or spheroids highly expressed cyclin D1, Bmi-1, and vimentin, and seldom expressed E-cadherin, while CD24+ or parental cells showed the opposite expression. Furthermore, cyclin D1-targeted small interfering RNA resulted in decreased vimentin expression in spheroids. Transfected cells also exhibited an obvious decrease in cell viability and migration, but an increase in cell apoptosis.
Conclusion: Cancer stem cell-like cells possess mesenchymal characteristics and EMT ability, and cyclin D1 involves in EMT mechanism, suggesting that EMT of cancer stem cell-like cells may play a key role in invasion and metastasis of ovarian cancer.
Keywords: cancer stem cell; cyclin D1; epithelial; mesenchymal transition; ovarian cancer.
Figures
Similar articles
-
Epithelial mesenchymal transition correlates with CD24+CD44+ and CD133+ cells in pancreatic cancer.Oncol Rep. 2012 May;27(5):1599-605. doi: 10.3892/or.2012.1681. Epub 2012 Feb 7. Oncol Rep. 2012. PMID: 22322379
-
CD24 expression is a marker for predicting clinical outcome and regulates the epithelial-mesenchymal transition in ovarian cancer via both the Akt and ERK pathways.Oncol Rep. 2017 Jun;37(6):3189-3200. doi: 10.3892/or.2017.5583. Epub 2017 Apr 19. Oncol Rep. 2017. PMID: 28440503 Free PMC article.
-
CD24⁺ ovary cancer cells exhibit an invasive mesenchymal phenotype.Biochem Biophys Res Commun. 2013 Mar 8;432(2):333-8. doi: 10.1016/j.bbrc.2013.01.102. Epub 2013 Feb 8. Biochem Biophys Res Commun. 2013. PMID: 23396061
-
PARP3 controls TGFβ and ROS driven epithelial-to-mesenchymal transition and stemness by stimulating a TG2-Snail-E-cadherin axis.Oncotarget. 2016 Sep 27;7(39):64109-64123. doi: 10.18632/oncotarget.11627. Oncotarget. 2016. PMID: 27579892 Free PMC article.
-
A Recipe for Successful Metastasis: Transition and Migratory Modes of Ovarian Cancer Cells.Cancers (Basel). 2024 Feb 15;16(4):783. doi: 10.3390/cancers16040783. Cancers (Basel). 2024. PMID: 38398174 Free PMC article. Review.
Cited by
-
Inhibition of cyclin D1 enhances sensitivity to radiotherapy and reverses epithelial to mesenchymal transition for esophageal cancer cells.Tumour Biol. 2016 Apr;37(4):5355-63. doi: 10.1007/s13277-015-4393-z. Epub 2015 Nov 11. Tumour Biol. 2016. PMID: 26561473
-
STON2 negatively modulates stem-like properties in ovarian cancer cells via DNMT1/MUC1 pathway.J Exp Clin Cancer Res. 2018 Dec 5;37(1):305. doi: 10.1186/s13046-018-0977-y. J Exp Clin Cancer Res. 2018. PMID: 30518424 Free PMC article.
-
Inhibitory effects of metformin at low concentration on epithelial-mesenchymal transition of CD44(+)CD117(+) ovarian cancer stem cells.Stem Cell Res Ther. 2015 Dec 30;6:262. doi: 10.1186/s13287-015-0249-0. Stem Cell Res Ther. 2015. PMID: 26718286 Free PMC article.
-
Circular RNA circZCCHC6 contributes to tumorigenesis by regulating LPCAT1 via miR-433-3p in non-small cell lung cancer.Clin Exp Med. 2022 Nov;22(4):647-659. doi: 10.1007/s10238-021-00780-2. Epub 2022 Jan 28. Clin Exp Med. 2022. PMID: 35089454
-
Selective Antitumor Activity of Datelliptium toward Medullary Thyroid Carcinoma by Downregulating RET Transcriptional Activity.Cancers (Basel). 2021 Jun 30;13(13):3288. doi: 10.3390/cancers13133288. Cancers (Basel). 2021. PMID: 34209165 Free PMC article.
References
-
- Nossov V, Amneus M, Su F, et al. The early detection of ovarian cancer: from traditional methods to proteomics. Can we really do better than serum CA-125? Am J Obstet Gynecol. 2008;199(3):215–223. - PubMed
-
- Guzmán-Ramírez N, Völler M, Wetterwald A, et al. In vitro propagation and characterization of neoplastic stem/progenitor-like cells from human prostate cancer tissue. Prostate. 2009;69(15):1683–1693. - PubMed
-
- Zhu Z, Hao X, Yan M, et al. Cancer stem/progenitor cells are highly enriched in CD133+ CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126(9):2067–2078. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

