mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells
- PMID: 23821483
- PMCID: PMC3870029
- DOI: 10.1002/jbmr.2031
mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells
Abstract
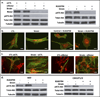
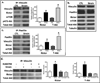
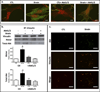
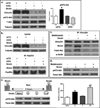
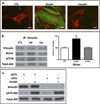
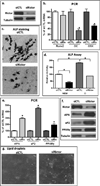
The cell cytoskeleton interprets and responds to physical cues from the microenvironment. Applying mechanical force to mesenchymal stem cells induces formation of a stiffer cytoskeleton, which biases against adipogenic differentiation and toward osteoblastogenesis. mTORC2, the mTOR complex defined by its binding partner rictor, is implicated in resting cytoskeletal architecture and is activated by mechanical force. We asked if mTORC2 played a role in mechanical adaptation of the cytoskeleton. We found that during bi-axial strain-induced cytoskeletal restructuring, mTORC2 and Akt colocalize with newly assembled focal adhesions (FA). Disrupting the function of mTORC2, or that of its downstream substrate Akt, prevented mechanically induced F-actin stress fiber development. mTORC2 becomes associated with vinculin during strain, and knockdown of vinculin prevents mTORC2 activation. In contrast, mTORC2 is not recruited to the FA complex during its activation by insulin, nor does insulin alter cytoskeletal structure. Further, when rictor was knocked down, the ability of mesenchymal stem cells (MSC) to enter the osteoblastic lineage was reduced, and when cultured in adipogenic medium, rictor-deficient MSC showed accelerated adipogenesis. This indicated that cytoskeletal remodeling promotes osteogenesis over adipogenesis. In sum, our data show that mTORC2 is involved in stem cell responses to biophysical stimuli, regulating both signaling and cytoskeletal reorganization. As such, mechanical activation of mTORC2 signaling participates in mesenchymal stem cell lineage selection, preventing adipogenesis by preserving β-catenin and stimulating osteogenesis by generating a stiffer cytoskeleton.
Keywords: ADIPOCYTE; AKT; OSTEOBLAST; RICTOR; VINCULIN.
© 2014 American Society for Bone and Mineral Research.
Conflict of interest statement
Figures

Similar articles
-
Mechanically activated Fyn utilizes mTORC2 to regulate RhoA and adipogenesis in mesenchymal stem cells.Stem Cells. 2013 Nov;31(11):2528-37. doi: 10.1002/stem.1476. Stem Cells. 2013. PMID: 23836527 Free PMC article.
-
Mechanical regulation of glycogen synthase kinase 3β (GSK3β) in mesenchymal stem cells is dependent on Akt protein serine 473 phosphorylation via mTORC2 protein.J Biol Chem. 2011 Nov 11;286(45):39450-6. doi: 10.1074/jbc.M111.265330. Epub 2011 Sep 28. J Biol Chem. 2011. PMID: 21956113 Free PMC article.
-
LARG GEF and ARHGAP18 orchestrate RhoA activity to control mesenchymal stem cell lineage.Bone. 2018 Feb;107:172-180. doi: 10.1016/j.bone.2017.12.001. Epub 2017 Dec 5. Bone. 2018. PMID: 29208526 Free PMC article.
-
Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways.Tissue Eng Part B Rev. 2012 Dec;18(6):436-44. doi: 10.1089/ten.TEB.2012.0014. Epub 2012 Aug 6. Tissue Eng Part B Rev. 2012. PMID: 22741572 Free PMC article. Review.
-
Biomechanical, biophysical and biochemical modulators of cytoskeletal remodelling and emergent stem cell lineage commitment.Commun Biol. 2023 Jan 19;6(1):75. doi: 10.1038/s42003-022-04320-w. Commun Biol. 2023. PMID: 36658332 Free PMC article. Review.
Cited by
-
The Spectrum of Molecular Pathways in Gliomas-An Up-to-Date Review.Biomedicines. 2023 Aug 16;11(8):2281. doi: 10.3390/biomedicines11082281. Biomedicines. 2023. PMID: 37626776 Free PMC article. Review.
-
mTOR signaling in skeletal development and disease.Bone Res. 2018 Jan 30;6:1. doi: 10.1038/s41413-017-0004-5. eCollection 2018. Bone Res. 2018. PMID: 29423330 Free PMC article.
-
Effect of Polymeric Matrix Stiffness on Osteogenic Differentiation of Mesenchymal Stem/Progenitor Cells: Concise Review.Polymers (Basel). 2021 Aug 31;13(17):2950. doi: 10.3390/polym13172950. Polymers (Basel). 2021. PMID: 34502988 Free PMC article. Review.
-
Microtopographical cues promote peripheral nerve regeneration via transient mTORC2 activation.Acta Biomater. 2017 Sep 15;60:220-231. doi: 10.1016/j.actbio.2017.07.031. Epub 2017 Jul 25. Acta Biomater. 2017. PMID: 28754648 Free PMC article.
-
Targeting P38 Pathway Regulates Bony Formation via MSC Recruitment during Mandibular Distraction Osteogenesis in Rats.Int J Med Sci. 2016 Oct 1;13(10):783-789. doi: 10.7150/ijms.16663. eCollection 2016. Int J Med Sci. 2016. PMID: 27766028 Free PMC article.
References
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

