Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor
- PMID: 23818853
- PMCID: PMC3688554
- DOI: 10.1371/journal.ppat.1003452
Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor
Abstract
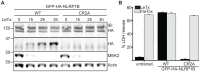
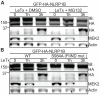
Inflammasomes are multimeric protein complexes that respond to infection by recruitment and activation of the Caspase-1 (CASP1) protease. Activated CASP1 initiates immune defense by processing inflammatory cytokines and by causing a rapid and lytic cell death called pyroptosis. Inflammasome formation is orchestrated by members of the nucleotide-binding domain and leucine-rich repeat (NLR) or AIM2-like receptor (ALR) protein families. Certain NLRs and ALRs have been shown to function as direct receptors for specific microbial ligands, such as flagellin or DNA, but the molecular mechanism responsible for activation of most NLRs is still poorly understood. Here we determine the mechanism of activation of the NLRP1B inflammasome in mice. NLRP1B, and its ortholog in rats, is activated by the lethal factor (LF) protease that is a key virulence factor secreted by Bacillus anthracis, the causative agent of anthrax. LF was recently shown to cleave mouse and rat NLRP1 directly. However, it is unclear if cleavage is sufficient for NLRP1 activation. Indeed, other LF-induced cellular events have been suggested to play a role in NLRP1B activation. Surprisingly, we show that direct cleavage of NLRP1B is sufficient to induce inflammasome activation in the absence of LF. Our results therefore rule out the need for other LF-dependent cellular effects in activation of NLRP1B. We therefore propose that NLRP1 functions primarily as a sensor of protease activity and thus could conceivably detect a broader spectrum of pathogens than just B. anthracis. By adding proteolytic cleavage to the previously established ligand-receptor mechanism of NLR activation, our results illustrate the remarkable flexibility with which the NLR architecture can be deployed for the purpose of pathogen-detection and host defense.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Functional degradation: A mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes.Science. 2019 Apr 5;364(6435):eaau1330. doi: 10.1126/science.aau1330. Epub 2019 Mar 14. Science. 2019. PMID: 30872533 Free PMC article.
-
Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function.Proc Natl Acad Sci U S A. 2014 Dec 2;111(48):17254-9. doi: 10.1073/pnas.1415756111. Epub 2014 Nov 17. Proc Natl Acad Sci U S A. 2014. PMID: 25404286 Free PMC article.
-
Bacillus anthracis induces NLRP3 inflammasome activation and caspase-8-mediated apoptosis of macrophages to promote lethal anthrax.Proc Natl Acad Sci U S A. 2022 Jan 11;119(2):e2116415119. doi: 10.1073/pnas.2116415119. Proc Natl Acad Sci U S A. 2022. PMID: 34996874 Free PMC article.
-
Anthrax and the inflammasome.Microbes Infect. 2012 May;14(5):392-400. doi: 10.1016/j.micinf.2011.12.005. Epub 2011 Dec 17. Microbes Infect. 2012. PMID: 22207185 Free PMC article. Review.
-
The NLRP1 inflammasomes.Immunol Rev. 2015 May;265(1):22-34. doi: 10.1111/imr.12283. Immunol Rev. 2015. PMID: 25879281 Review.
Cited by
-
The NLRP1 inflammasome attenuates colitis and colitis-associated tumorigenesis.J Immunol. 2015 Apr 1;194(7):3369-80. doi: 10.4049/jimmunol.1402098. Epub 2015 Feb 27. J Immunol. 2015. PMID: 25725098 Free PMC article.
-
The noncanonical inflammasome in health and disease.Infect Med (Beijing). 2022 Sep 11;1(3):208-216. doi: 10.1016/j.imj.2022.09.001. eCollection 2022 Sep. Infect Med (Beijing). 2022. PMID: 38077630 Free PMC article. Review.
-
Activation and regulation mechanisms of NOD-like receptors based on structural biology.Front Immunol. 2022 Sep 15;13:953530. doi: 10.3389/fimmu.2022.953530. eCollection 2022. Front Immunol. 2022. PMID: 36189327 Free PMC article. Review.
-
Protective and detrimental roles of inflammasomes in disease.Semin Immunopathol. 2015 Jul;37(4):313-22. doi: 10.1007/s00281-015-0485-5. Epub 2015 Apr 21. Semin Immunopathol. 2015. PMID: 25895577 Review.
-
Bacterial Toxin and Effector Regulation of Intestinal Immune Signaling.Front Cell Dev Biol. 2022 Feb 16;10:837691. doi: 10.3389/fcell.2022.837691. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35252199 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

