Transorganellar complementation redefines the biochemical continuity of endoplasmic reticulum and chloroplasts
- PMID: 23818635
- PMCID: PMC3718160
- DOI: 10.1073/pnas.1306331110
Transorganellar complementation redefines the biochemical continuity of endoplasmic reticulum and chloroplasts
Abstract
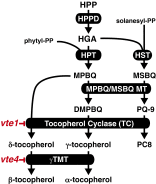
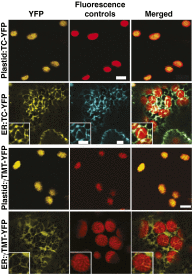
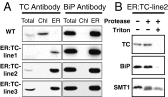
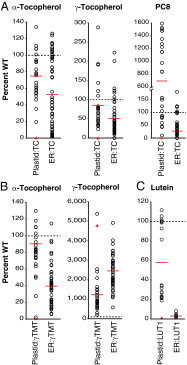
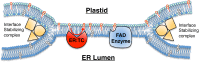
Tocopherols are nonpolar compounds synthesized and localized in plastids but whose genetic elimination specifically impacts fatty acid desaturation in the endoplasmic reticulum (ER), suggesting a direct interaction with ER-resident enzymes. To functionally probe for such interactions, we developed transorganellar complementation, where mutated pathway activities in one organelle are experimentally tested for substrate accessibility and complementation by active enzymes retargeted to a companion organelle. Mutations disrupting three plastid-resident activities in tocopherol and carotenoid synthesis were complemented from the ER in this fashion, demonstrating transorganellar access to at least seven nonpolar, plastid envelope-localized substrates from the lumen of the ER, likely through plastid:ER membrane interaction domains. The ability of enzymes in either organelle to access shared, nonpolar plastid metabolite pools redefines our understanding of the biochemical continuity of the ER and chloroplast with profound implications for the integration and regulation of organelle-spanning pathways that synthesize nonpolar metabolites in plants.
Keywords: MAM; PLAM; hemifusion; metabolism; vitamin E.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Redefining the metabolic continuity of chloroplasts and ER.Trends Plant Sci. 2014 Aug;19(8):501-7. doi: 10.1016/j.tplants.2014.02.013. Epub 2014 Mar 26. Trends Plant Sci. 2014. PMID: 24679997 Review.
-
A role for lipid trafficking in chloroplast biogenesis.Prog Lipid Res. 2008 Sep;47(5):381-9. doi: 10.1016/j.plipres.2008.04.001. Epub 2008 Apr 7. Prog Lipid Res. 2008. PMID: 18440317 Review.
-
Lipid trafficking between the endoplasmic reticulum and the plastid in Arabidopsis requires the extraplastidic TGD4 protein.Plant Cell. 2008 Aug;20(8):2190-204. doi: 10.1105/tpc.108.061176. Epub 2008 Aug 8. Plant Cell. 2008. PMID: 18689504 Free PMC article.
-
Mutations of the ER to plastid lipid transporters TGD1, 2, 3 and 4 and the ER oleate desaturase FAD2 suppress the low temperature-induced phenotype of Arabidopsis tocopherol-deficient mutant vte2.Plant J. 2010 Jun 1;62(6):1004-18. doi: 10.1111/j.1365-313X.2010.04212.x. Epub 2010 Mar 25. Plant J. 2010. PMID: 20345604
-
Lipid transport mediated by Arabidopsis TGD proteins is unidirectional from the endoplasmic reticulum to the plastid.Plant Cell Physiol. 2010 Jun;51(6):1019-28. doi: 10.1093/pcp/pcq053. Epub 2010 Apr 21. Plant Cell Physiol. 2010. PMID: 20410050
Cited by
-
Organelle Interactions in Plant Cells.Results Probl Cell Differ. 2024;73:43-69. doi: 10.1007/978-3-031-62036-2_3. Results Probl Cell Differ. 2024. PMID: 39242374 Review.
-
Monitoring calcium handling by the plant endoplasmic reticulum with a low-Ca2+ -affinity targeted aequorin reporter.Plant J. 2022 Feb;109(4):1014-1027. doi: 10.1111/tpj.15610. Epub 2021 Dec 11. Plant J. 2022. PMID: 34837294 Free PMC article.
-
Trafficking of proteins through plastid stromules.Plant Cell. 2013 Aug;25(8):2774-82. doi: 10.1105/tpc.113.112870. Epub 2013 Aug 27. Plant Cell. 2013. PMID: 23983219 Free PMC article. Review.
-
Identification and Characterization of Sterol Acyltransferases Responsible for Steryl Ester Biosynthesis in Tomato.Front Plant Sci. 2018 May 8;9:588. doi: 10.3389/fpls.2018.00588. eCollection 2018. Front Plant Sci. 2018. PMID: 29868054 Free PMC article.
-
The Puzzle of Metabolite Exchange and Identification of Putative Octotrico Peptide Repeat Expression Regulators in the Nascent Photosynthetic Organelles of Paulinella chromatophora.Front Microbiol. 2020 Nov 27;11:607182. doi: 10.3389/fmicb.2020.607182. eCollection 2020. Front Microbiol. 2020. PMID: 33329499 Free PMC article.
References
-
- Soll J, Schultz G, Joyard J, Douce R, Block MA. Localization and synthesis of prenylquinones in isolated outer and inner envelope membranes from spinach chloroplasts. Arch Biochem Biophys. 1985;238(1):290–299. - PubMed
-
- Joyard J, et al. Chloroplast proteomics and the compartmentation of plastidial isoprenoid biosynthetic pathways. Mol Plant. 2009;2(6):1154–1180. - PubMed
-
- Lichtenthaler HK, Prenzel U, Douce R, Joyard J. Localization of prenylquinones in the envelope of spinach chloroplasts. Biochim Biophys Acta. 1981;641(1):99–105. - PubMed
-
- Maeda H, DellaPenna D. Tocopherol functions in photosynthetic organisms. Curr Opin Plant Biol. 2007;10(3):260–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

