Adenosine-A3 receptors in neutrophil microdomains promote the formation of bacteria-tethering cytonemes
- PMID: 23817552
- PMCID: PMC3736131
- DOI: 10.1038/embor.2013.89
Adenosine-A3 receptors in neutrophil microdomains promote the formation of bacteria-tethering cytonemes
Abstract
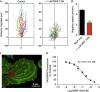
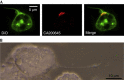
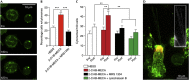
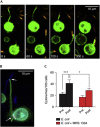
The A3-adenosine receptor (A3AR) has recently emerged as a key regulator of neutrophil behaviour. Using a fluorescent A3AR ligand, we show that A3ARs aggregate in highly polarized immunomodulatory microdomains on human neutrophil membranes. In addition to regulating chemotaxis, A3ARs promote the formation of filipodia-like projections (cytonemes) that can extend up to 100 μm to tether and 'reel in' pathogens. Exposure to bacteria or an A3AR agonist stimulates the formation of these projections and bacterial phagocytosis, whereas an A3AR-selective antagonist inhibits cytoneme formation. Our results shed new light on the behaviour of neutrophils and identify the A3AR as a potential target for modulating their function.
Conflict of interest statement
S.J.H. and B.K. are founders and directors of the University of Nottingham spin-out company that provided CA20065.
Figures
Similar articles
-
Kinetic analysis of antagonist-occupied adenosine-A3 receptors within membrane microdomains of individual cells provides evidence of receptor dimerization and allosterism.FASEB J. 2014 Oct;28(10):4211-22. doi: 10.1096/fj.13-247270. Epub 2014 Jun 26. FASEB J. 2014. PMID: 24970394 Free PMC article.
-
Pharmacological characterization of DPTN and other selective A3 adenosine receptor antagonists.Purinergic Signal. 2021 Dec;17(4):737-746. doi: 10.1007/s11302-021-09823-5. Epub 2021 Oct 28. Purinergic Signal. 2021. PMID: 34713378 Free PMC article.
-
ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors.Science. 2006 Dec 15;314(5806):1792-5. doi: 10.1126/science.1132559. Science. 2006. PMID: 17170310
-
Species dependence of A3 adenosine receptor pharmacology and function.Purinergic Signal. 2023 Sep;19(3):523-550. doi: 10.1007/s11302-022-09910-1. Epub 2022 Dec 20. Purinergic Signal. 2023. PMID: 36538251 Free PMC article. Review.
-
Current Status in the Design and Development of Agonists and Antagonists of Adenosine A3 Receptor as Potential Therapeutic Agents.Curr Pharm Des. 2019;25(25):2772-2787. doi: 10.2174/1381612825666190716114056. Curr Pharm Des. 2019. PMID: 31333098 Review.
Cited by
-
Adenosine and Inflammation: Here, There and Everywhere.Int J Mol Sci. 2021 Jul 19;22(14):7685. doi: 10.3390/ijms22147685. Int J Mol Sci. 2021. PMID: 34299305 Free PMC article. Review.
-
Cytonemes Versus Neutrophil Extracellular Traps in the Fight of Neutrophils with Microbes.Int J Mol Sci. 2020 Jan 16;21(2):586. doi: 10.3390/ijms21020586. Int J Mol Sci. 2020. PMID: 31963289 Free PMC article. Review.
-
Fluorescent ligands: Bringing light to emerging GPCR paradigms.Br J Pharmacol. 2020 Mar;177(5):978-991. doi: 10.1111/bph.14953. Epub 2020 Feb 6. Br J Pharmacol. 2020. PMID: 31877233 Free PMC article. Review.
-
High PANX1 Expression Leads to Neutrophil Recruitment and the Formation of a High Adenosine Immunosuppressive Tumor Microenvironment in Basal-like Breast Cancer.Cancers (Basel). 2022 Jul 11;14(14):3369. doi: 10.3390/cancers14143369. Cancers (Basel). 2022. PMID: 35884429 Free PMC article.
-
Rhes Tunnels: A Radical New Way of Communication in the Brain's Striatum?Bioessays. 2020 Jun;42(6):e1900231. doi: 10.1002/bies.201900231. Epub 2020 Apr 1. Bioessays. 2020. PMID: 32236969 Free PMC article. Review.
References
-
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314: 1792–1795 - PubMed
-
- Kronlage M et al. (2010) Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal 3: ra55. - PubMed
-
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CCM, Beck PL, Muruve DA, Kubes P (2010) Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330: 362–366 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

