Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression
- PMID: 23816887
- PMCID: PMC3753845
- DOI: 10.1128/MCB.00217-13
Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression
Abstract
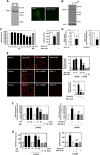
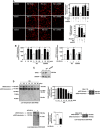
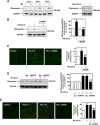
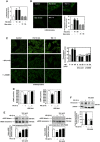
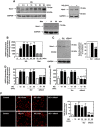
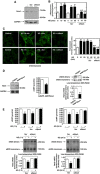
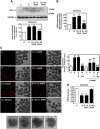
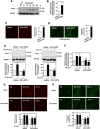
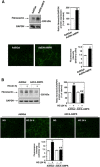
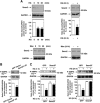
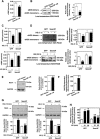
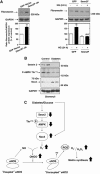
Mesangial matrix accumulation is an early feature of glomerular pathology in diabetes. Oxidative stress plays a critical role in hyperglycemia-induced glomerular injury. Here, we demonstrate that, in glomerular mesangial cells (MCs), endothelial nitric oxide synthase (eNOS) is uncoupled upon exposure to high glucose (HG), with enhanced generation of reactive oxygen species (ROS) and decreased production of nitric oxide. Peroxynitrite mediates the effects of HG on eNOS dysfunction. HG upregulates Nox4 protein, and inhibition of Nox4 abrogates the increase in ROS and peroxynitrite generation, as well as the eNOS uncoupling triggered by HG, demonstrating that Nox4 functions upstream from eNOS. Importantly, this pathway contributes to HG-induced MC fibronectin accumulation. Nox4-mediated eNOS dysfunction was confirmed in glomeruli of a rat model of type 1 diabetes. Sestrin 2-dependent AMP-activated protein kinase (AMPK) activation attenuates HG-induced MC fibronectin synthesis through blockade of Nox4-dependent ROS and peroxynitrite generation, with subsequent eNOS uncoupling. We also find that HG negatively regulates sestrin 2 and AMPK, thereby promoting Nox4-mediated eNOS dysfunction and increased fibronectin. These data identify a protective function for sestrin 2/AMPK and potential targets for intervention to prevent fibrotic injury in diabetes.
Figures

Similar articles
-
Nox4 NADPH oxidase mediates peroxynitrite-dependent uncoupling of endothelial nitric-oxide synthase and fibronectin expression in response to angiotensin II: role of mitochondrial reactive oxygen species.J Biol Chem. 2013 Oct 4;288(40):28668-86. doi: 10.1074/jbc.M113.470971. Epub 2013 Aug 12. J Biol Chem. 2013. PMID: 23940049 Free PMC article.
-
Glycated human serum albumin induces NF-κB activation and endothelial nitric oxide synthase uncoupling in human umbilical vein endothelial cells.J Diabetes Complications. 2015 Nov-Dec;29(8):984-92. doi: 10.1016/j.jdiacomp.2015.07.016. Epub 2015 Jul 18. J Diabetes Complications. 2015. PMID: 26297216
-
Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression.J Biol Chem. 2008 Aug 29;283(35):24061-76. doi: 10.1074/jbc.M803964200. Epub 2008 Jun 16. J Biol Chem. 2008. PMID: 18559349 Free PMC article.
-
Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS?Int J Mol Sci. 2019 Aug 2;20(15):3775. doi: 10.3390/ijms20153775. Int J Mol Sci. 2019. PMID: 31382355 Free PMC article. Review.
-
S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling.Antioxid Redox Signal. 2011 May 15;14(10):1769-75. doi: 10.1089/ars.2011.3904. Epub 2011 Mar 27. Antioxid Redox Signal. 2011. PMID: 21261471 Free PMC article. Review.
Cited by
-
Exogenous Nucleotides Ameliorate Insulin Resistance Induced by Palmitic Acid in HepG2 Cells through the IRS-1/AKT/FOXO1 Pathways.Nutrients. 2024 Jun 7;16(12):1801. doi: 10.3390/nu16121801. Nutrients. 2024. PMID: 38931156 Free PMC article.
-
Locked in Structure: Sestrin and GATOR-A Billion-Year Marriage.Cells. 2024 Sep 21;13(18):1587. doi: 10.3390/cells13181587. Cells. 2024. PMID: 39329768 Free PMC article. Review.
-
Biochemical Basis of Sestrin Physiological Activities.Trends Biochem Sci. 2016 Jul;41(7):621-632. doi: 10.1016/j.tibs.2016.04.005. Epub 2016 May 10. Trends Biochem Sci. 2016. PMID: 27174209 Free PMC article. Review.
-
Effects of cellular senescence on metabolic pathways in non-immune and immune cells.Mech Ageing Dev. 2021 Mar;194:111428. doi: 10.1016/j.mad.2020.111428. Epub 2020 Dec 28. Mech Ageing Dev. 2021. PMID: 33383073 Free PMC article. Review.
-
Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition.Antioxidants (Basel). 2020 Jul 2;9(7):573. doi: 10.3390/antiox9070573. Antioxidants (Basel). 2020. PMID: 32630636 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
