Cooperativity between MAPK and PI3K signaling activation is required for glioblastoma pathogenesis
- PMID: 23814263
- PMCID: PMC3779038
- DOI: 10.1093/neuonc/not084
Cooperativity between MAPK and PI3K signaling activation is required for glioblastoma pathogenesis
Abstract
Background: Glioblastoma (GBM) genomes feature recurrent genetic alterations that dysregulate core intracellular signaling pathways, including the G1/S cell cycle checkpoint and the MAPK and PI3K effector arms of receptor tyrosine kinase (RTK) signaling. Elucidation of the phenotypic consequences of activated RTK effectors is required for the design of effective therapeutic and diagnostic strategies.
Methods: Genetically defined, G1/S checkpoint-defective cortical murine astrocytes with constitutively active Kras and/or Pten deletion mutations were used to systematically investigate the individual and combined roles of these 2 RTK signaling effectors in phenotypic hallmarks of glioblastoma pathogenesis, including growth, migration, and invasion in vitro. A novel syngeneic orthotopic allograft model system was used to examine in vivo tumorigenesis.
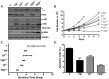
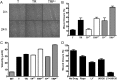
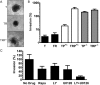
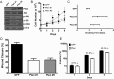
Results: Constitutively active Kras and/or Pten deletion mutations activated both MAPK and PI3K signaling. Their combination led to maximal growth, migration, and invasion of G1/S-defective astrocytes in vitro and produced progenitor-like transcriptomal profiles that mimic human proneural GBM. Activation of both RTK effector arms was required for in vivo tumorigenesis and produced highly invasive, proneural-like GBM.
Conclusions: These results suggest that cortical astrocytes can be transformed into GBM and that combined dysregulation of MAPK and PI3K signaling revert G1/S-defective astrocytes to a primitive gene expression state. This genetically-defined, immunocompetent model of proneural GBM will be useful for preclinical development of MAPK/PI3K-targeted, subtype-specific therapies.
Keywords: Pten; astrocytes; genetically engineered mouse; glioblastoma; invasion.
Figures


Similar articles
-
Combination therapy with potent PI3K and MAPK inhibitors overcomes adaptive kinome resistance to single agents in preclinical models of glioblastoma.Neuro Oncol. 2017 Oct 19;19(11):1469-1480. doi: 10.1093/neuonc/nox044. Neuro Oncol. 2017. PMID: 28379424 Free PMC article.
-
Core pathway mutations induce de-differentiation of murine astrocytes into glioblastoma stem cells that are sensitive to radiation but resistant to temozolomide.Neuro Oncol. 2016 Jul;18(7):962-73. doi: 10.1093/neuonc/nov321. Epub 2016 Jan 28. Neuro Oncol. 2016. PMID: 26826202 Free PMC article.
-
A preclinical orthotopic model for glioblastoma recapitulates key features of human tumors and demonstrates sensitivity to a combination of MEK and PI3K pathway inhibitors.Dis Model Mech. 2015 Jan;8(1):45-56. doi: 10.1242/dmm.018168. Epub 2014 Nov 27. Dis Model Mech. 2015. PMID: 25431423 Free PMC article.
-
Targeting strategies on miRNA-21 and PDCD4 for glioblastoma.Arch Biochem Biophys. 2015 Aug 15;580:64-74. doi: 10.1016/j.abb.2015.07.001. Epub 2015 Jul 2. Arch Biochem Biophys. 2015. PMID: 26142886 Review.
-
PTEN signaling pathways in glioblastoma.Cancer Biol Ther. 2008 Sep;7(9):1321-5. doi: 10.4161/cbt.7.9.6954. Epub 2008 Sep 8. Cancer Biol Ther. 2008. PMID: 18836294 Review.
Cited by
-
Myocyte enhancer factor 2D promotes tumorigenicity in malignant glioma cells.Tumour Biol. 2016 Jan;37(1):601-10. doi: 10.1007/s13277-015-3791-6. Epub 2015 Aug 4. Tumour Biol. 2016. PMID: 26234765
-
Ras-mediated modulation of pyruvate dehydrogenase activity regulates mitochondrial reserve capacity and contributes to glioblastoma tumorigenesis.Neuro Oncol. 2015 Sep;17(9):1220-30. doi: 10.1093/neuonc/nou369. Epub 2015 Feb 23. Neuro Oncol. 2015. PMID: 25712957 Free PMC article.
-
Identification of TNFAIP6 as a hub gene associated with the progression of glioblastoma by weighted gene co-expression network analysis.IET Syst Biol. 2022 Sep;16(5):145-156. doi: 10.1049/syb2.12046. Epub 2022 Jun 29. IET Syst Biol. 2022. PMID: 35766985 Free PMC article.
-
Selective CREB-dependent cyclin expression mediated by the PI3K and MAPK pathways supports glioma cell proliferation.Oncogenesis. 2014 Jun 30;3(6):e108. doi: 10.1038/oncsis.2014.21. Oncogenesis. 2014. PMID: 24979279 Free PMC article.
-
Intrinsic Astrocyte Heterogeneity Influences Tumor Growth in Glioma Mouse Models.Brain Pathol. 2017 Jan;27(1):36-50. doi: 10.1111/bpa.12348. Epub 2016 Apr 13. Brain Pathol. 2017. PMID: 26762242 Free PMC article.
References
-
- CBTRUS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2008. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2012.
-
- Gaspar LE, Fisher BJ, Macdonald DR, et al. Supratentorial malignant glioma: patterns of recurrence and implications for external beam local treatment. Int J Radiat Oncol Biol Phys. 1992;24:55–57. - PubMed
-
- Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

