Maintenance chemotherapy for ovarian cancer
- PMID: 23813336
- PMCID: PMC6457821
- DOI: 10.1002/14651858.CD007414.pub3
Maintenance chemotherapy for ovarian cancer
Abstract
Background: Epithelial ovarian cancer accounts for about 90% of all cases of ovarian cancer. Debulking surgery and six courses of platinum-based chemotherapy results in complete clinical remission (CCR) in up to 75% of cases. However, 75% of the responders will relapse within a median time of 18 to 28 months and only 20% to 40% of women will survive beyond five years. It has been suggested that maintenance chemotherapy could assist in prolonging remission. To date, there has not been a systematic review on the impact of maintenance chemotherapy for epithelial ovarian cancer.
Objectives: To assess the effectiveness and toxicity of maintenance chemotherapy for epithelial ovarian cancer and to evaluate the impact on quality of life (QoL).
Search methods: In the original review we searched the Cochrane Gynaecological Cancer Review Group Specialised Register, The Cochrane Central Register of Controlled Trails (CENTRAL, The Cochrane Library 2009, Issue 1), MEDLINE, EMBASE, PubMed, CBMdisc, CNKI and VIP (to May 2009). We collected information from ongoing trials, checked reference lists of published articles and consulted experts in the field. For this update, the searches were extended to October 2012.
Selection criteria: Randomised controlled trials (RCTs) comparing maintenance chemotherapy with no further intervention, maintenance radiotherapy or other maintenance therapy.
Data collection and analysis: Two review authors independently assessed trials for eligibility and quality and extracted data. We analysed overall survival (OS) and progression-free survival (PFS) rates as dichotomous variables. Toxicity and QoL data were extracted where present. All analyses were based on intention-to-treat (ITT) on the endpoint of survival. We also analysed data by subgroups of drugs.
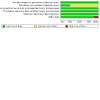
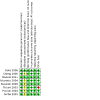
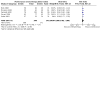
Main results: We included eight trials (1644 women). When all chemotherapy regimens were combined, meta-analysis indicated no significant difference in three-, five- and 10-year OS or PFS. For five-year OS, the combined risk ratio (RR) was 1.03 (95% confidence interval (CI) 0.96 to 1.10) and for the five-year PFS, the combined RR was 1.06 (95% CI 0.97 to 1.17). Results were very similar when trials of different regimens were analysed. Comparing chemotherapy with radiotherapy, only the RR for 10-year PFS in pathological complete remission (PCR) was in favour of whole abdominal radiotherapy 0.51 (95% CI 0.27 to 1.00), while three- and five-year OS rates have no significant difference between the two groups.
Authors' conclusions: There is no evidence to suggest that the use of platinum agents, doxorubicin or paclitaxel used as maintenance chemotherapy is more effective than observation alone. Further investigations regarding the effect of paclitaxel used as maintenance chemotherapy are required.
Conflict of interest statement
None known.
Figures
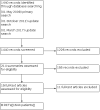



















Update of
-
Maintenance chemotherapy for ovarian cancer.Cochrane Database Syst Rev. 2010 Sep 8;(9):CD007414. doi: 10.1002/14651858.CD007414.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2013 Jun 29;(6):CD007414. doi: 10.1002/14651858.CD007414.pub3. PMID: 20824860 Updated. Review.
Similar articles
-
Maintenance chemotherapy for ovarian cancer.Cochrane Database Syst Rev. 2010 Sep 8;(9):CD007414. doi: 10.1002/14651858.CD007414.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2013 Jun 29;(6):CD007414. doi: 10.1002/14651858.CD007414.pub3. PMID: 20824860 Updated. Review.
-
Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer.Cochrane Database Syst Rev. 2015 Dec 17;2015(12):CD004706. doi: 10.1002/14651858.CD004706.pub5. Cochrane Database Syst Rev. 2015. PMID: 26676202 Free PMC article. Review.
-
Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer.Cochrane Database Syst Rev. 2012 Aug 15;2012(8):CD005343. doi: 10.1002/14651858.CD005343.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2019 Oct 31;2019(10). doi: 10.1002/14651858.CD005343.pub4. PMID: 22895947 Free PMC article. Updated. Review.
-
Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer.Cochrane Database Syst Rev. 2012 Mar 14;3(3):CD004706. doi: 10.1002/14651858.CD004706.pub4. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2015 Dec 17;(12):CD004706. doi: 10.1002/14651858.CD004706.pub5. PMID: 22419298 Free PMC article. Updated. Review.
-
Interval debulking surgery for advanced epithelial ovarian cancer.Cochrane Database Syst Rev. 2010 Oct 6;(10):CD006014. doi: 10.1002/14651858.CD006014.pub5. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2013 Apr 30;(4):CD006014. doi: 10.1002/14651858.CD006014.pub6. PMID: 20927744 Updated. Review.
Cited by
-
A comprehensive survey into the role of microRNAs in ovarian cancer chemoresistance; an updated overview.J Ovarian Res. 2022 Jul 7;15(1):81. doi: 10.1186/s13048-022-01012-1. J Ovarian Res. 2022. PMID: 35799305 Free PMC article. Review.
-
Functional characterization of a novel transcript of ERCC1 in chemotherapy resistance of ovarian cancer.Oncotarget. 2017 Aug 24;8(49):85759-85771. doi: 10.18632/oncotarget.20482. eCollection 2017 Oct 17. Oncotarget. 2017. PMID: 29156754 Free PMC article.
-
Clinical outcomes in ovarian cancer patients receiving three versus more cycles of chemotherapy after neoadjuvant treatment and interval cytoreductive surgery.Int J Gynecol Cancer. 2019 Sep;29(7):1156-1163. doi: 10.1136/ijgc-2019-000374. Epub 2019 Jul 27. Int J Gynecol Cancer. 2019. PMID: 31352365 Free PMC article.
-
Efficacy of pegylated liposomal doxorubicin maintenance therapy in platinum-sensitive recurrent epithelial ovarian cancer: a retrospective study.Arch Gynecol Obstet. 2019 Jun;299(6):1641-1649. doi: 10.1007/s00404-019-05104-0. Epub 2019 Mar 1. Arch Gynecol Obstet. 2019. PMID: 30824986 Free PMC article.
-
Correlation of maintenance chemotherapy and improved survival in patients with locally advanced unresectable pancreatic head adenocarcinoma receiving neoadjuvant chemotherapy and concurrent chemoradiotherapy.Am J Cancer Res. 2024 May 15;14(5):2313-2325. doi: 10.62347/AGTB1099. eCollection 2024. Am J Cancer Res. 2024. PMID: 38859863 Free PMC article.
References
References to studies included in this review
Bolis 2006 {published data only}
-
- Bolis G, Danese S, Tateo S, Rabaiotti E, D'Agostino G, Merisio C, et al.Epidoxorubicin versus no treatment as consolidation therapy in advanced ovarian cancer:results from a phase IIstudy. International Journal of Gynecology Cancer 2006;16(suppl 1):74-8. - PubMed
Cheng 2006 {published data only}
-
- Cheng NH, Huang HF, Pan LY, Shen K, Wu M, Yang JX.Consolidation chemotherapy in advanced epithelial ovarian carcinoma:a prospective randomized controlled trial. Medical Journal of Chinese People's Liberation Army 2006;31(7):654-6.
Mannel 2011 {published data only}
-
- Mannel RS, Brady MF, Kohn EC, Hanjani P, Hiura M, Lee R, et al.A randomized phase III trial of IV carboplatin and paclitaxel × 3 courses followed by observation versus weekly maintenance low-dose paclitaxel in patients with early-stage ovarian carcinoma: a Gynecologic Oncology Group Study. Gynecology Oncology 2011;122(1):89-94. - PMC - PubMed
Nicoletto 2004 {published data only}
Pecorelli 2009 {published data only}
-
- Pecorelli S, Favalli G, Gadducci A, Katsaros D, Panici PB, Carpi A, et al, After 6 Italian Cooperative Group.Phase III trial of observation versus six courses of paclitaxel in patients with advanced epithelial ovarian cancer in complete response after six courses of paclitaxel/platinum-based chemotherapy: final results of the After-6 protocol 1. Journal of Clinical Oncology 2009;27(28):4642-8. - PubMed
Piccart 2003 {published and unpublished data}
-
- Piccart MJ, Floquet A, Scarfone G, Willemse PHB, Emerich J, Vergote I, et al.Intraperitoneal cisplatin versus no further treatment: 8-year results of EORTC 55875, a randomized phase 3 study in ovarian cancer patients with a pathologically complete remission after platinum-based intravenous chemotherapy. International Journal of Gynecologic Cancer 2003;13(suppl 2):196-203. - PubMed
Placido 2004 {published and unpublished data}
-
- Placido SD, Scambia G, Vagno GD, Naglieri E, Lombardi VA, Biamonte R, et al.Toptecan compared with no therapy after response to surgery and carboplatin/paclitaxel in patients with ovarian cancer: Multicenter Italian Trials in Ovarian Cancer (MITO-1) randomized study. Journal of Clinical Oncology 2004;22(13):2635-42. - PubMed
Sorbe 2003 {published data only}
-
- Sorbe B.Consolidation treatment of advanced (FIGO stage 3) ovarian carcinoma in complete surgical remission after induction chemotherapy: A randomized, controlled, clinical trial comparing whole abdominal radiotherapy, chemotherapy, and no further treatment. International Journal of Gynecologic Cancer 2003;13(3):278-86. - PubMed
References to studies excluded from this review
Abaid 2010 {published data only}
-
- Abaid LN, Goldstein BH, Micha JP, Rettenmaier MA, Brown JV, Markman M.Improved overall survival with 12 cycles of single-agent paclitaxel maintenance therapy following a complete response to induction chemotherapy in advanced ovarian carcinoma. Oncology 2010;78(5-6):389-93. - PubMed
Bois 2014 {published data only}
-
- Bois A, Floquet A, Kim JW, Rau J, Campo JM, Friedlander M, et al.Incorporation of Pazopanib in maintenance therapy of ovarian cancer. Journal of Clinical Oncology 2014;32(30):3374-87. - PubMed
Cure 2001 {published data only}
-
- Cure H, Battista C, Guastalla JP, Fabbro M, Tubiana-Mathieu N, Bourgeois H, et al.Phase 3 randomized trial of high-dose chemotherapy (HDC) and peripheral blood stem cell (PBSC) support as consolidation in patients with advanced ovarian cancer (AOC): 5-year follow-up of a GINECO/FNCLCC/SFGM-TC/Italy study. In: Proceedings of American Society of Clinical Oncology. 2001.
Gordon 2011 {published data only}
-
- Gordon AN, Teneriello M, Janicek MF, Hines J, Lim PC, Chen MD, et al.Phase III trial of induction gemcitabine or paclitaxel plus carboplatin followedby paclitaxel consolidation in ovarian cancer. Gynecologic Oncology 2011;123:479-85. - PubMed
Lee 2006 {published data only}
-
- Lee SJ, Lee JW, Min JA, Park CS, Kim BG, Lee JH, et al.A pilot study of three-cycle consolidation chemotherapy with paclitaxel and platinum inepithelial ovarian cancer patients with clinical complete response after paclitaxel and platinum chemotherapy. International Journal of Gynecological Cancer 2006;16(1):95-100. - PubMed
Lesnock 2011 {published data only}
Mannel 2010 {published data only}
-
- Mannel R, Brady M, Kohn E, Hanjani P, Hiura M, Lee R, et al. In:Gynecologic Oncology.Conference: 41st Annual Meeting of the Society of Gynecologic Oncologists, SGO San Francisco, CA United States. Vol. 116. March 2010:S2.
Markman 2003 {published data only}
-
- Markman M, Liu PY, Wilczynski S, Mank B, Copeland LJ, Alvarez RD, et al.Phase 3 randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: A Southwest Oncology Group and Gynecologic Oncology Group Trial. Journal of Clinical Oncology 2003;21(13):2460-5. - PubMed
Markman 2009 {published data only}
-
- Markman M, Liu PY, Moon J, Monk BJ, Copeland L, Wilczynski S, et al.Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel: follow-up of a Southwest Oncology Group and Gynecologic Oncology Group phase 3 trial. Gynecology Oncology 2009;114(2):195-8. - PMC - PubMed
Scarfone 2002 {published data only}
-
- Scarfone G, Merisio C, Garavaglia E, Richiardi G, Tateo S, D'Agostino S, et al.A phase 3 trial of consolidation versus NIHL (NIL) for advanced epithelial ovarian cancer (AEOC) after complete remission (CR). In: Proceedings of American Society of Clinical Oncology. 2002.
Suidan 2014 {published data only}
-
- Suidan RS, Clair CM, Lee SJ, Barlin JN, Roche KL, Tanner EJ, et al.A comparison of primary intraperitoneal chemotherapy to consolidation intraperitoneal chemotherapy in optimally resected advancedovarian cancer. Gycecologic Oncology 2014;134(3):468-72. - PubMed
References to ongoing studies
NCT00108745 {published data only}
-
- NCT00108745.Paclitaxel or polyglutamate paclitaxel or observation in treating women with stage III or stage IV ovarian epithelial or peritoneal cancer. http://clinicaltrials.gov/show/NCT00108745.
Additional references
Bertelsen 1993
-
- Bertelsen K, Jakobsen A, Strøyer J, Nielsen K, Sandberg E, Andersen JE, et al.A prospective randomized comparison of 6 and 12 cycles of cyclophosphamide, adriamycin, and cisplatin in advanced epithelial ovarian cancer: a Danish Ovarian Study Group trial (DACOVA). Gynecologic Oncology 1993;49(1):30-6. - PubMed
GLOBOCAN 2002
-
- Ferlay J, Bray F, Pisani P, Parkin DM.GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase 2004; 2004;(No.5. version 2.0).
GLOBOCAN 2012
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al.GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on 27/11/2017.
Hakes 1992
-
- Hakes TB, Chalas E, Hoskins WJ, Jones WB, Markman M, Rubin SC.Randomized prospective trial of 5 versus 10 cycles of cyclophosphamide doxorubicin and cisplatin in advanced ovarian carcinoma. Gynecologic Oncology 1992;45(3):284-8. - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG.Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Kikuchi 2005
-
- Kikuchi Y, Kita T, Takano M, Kudoh K, Yamamoto K.Treatment options in the management of ovarian cancer. Expert Opinion on Pharmacotherapy 2005;6(5):743-54. - PubMed
Lambert 1997
-
- Lambert HE, Rustin GJS, Gregory WM, Nelstrop AE.A randomized trial of five versus eight courses of cisplatin or carboplatin in advanced epithelial ovarian carcinoma. Annals of Oncology 1997;8:327-33. - PubMed
Langendam 2013
Meader 2014
Ozols 2004
-
- Ozols RF.Maintenance chemotherapy standard care. In: American Society of Clinical Oncology. 2004.
Ozols 2006
-
- Ozols RF.Challenges for chemotherapy in ovarian cancer. Annals of Oncology 2006;17(suppl.5):181-7. - PubMed
Poveda 2003
-
- Poveda A.Ovarian cancer treatment: what is new. International Journal of Gynecologic Cancer 2003;13(suppl 2):241-50. - PubMed
Stuart 2003
-
- Stuart GCE.First-line treatment regimens and the role of consolidation therapy in advanced ovarian cancer. Gynecologic Oncology 2003;90:8-15. - PubMed
Thigpen 2004
-
- Thigpen T.First-line therapy for ovarian carcinoma: What's next? Cancer Investigation 2004;22(suppl 2):21-8. - PubMed
Varia 2003
-
- Varia MA, Stehman FB, Bundy BN, Benda JA, Clarke-Pearson DL, Alvarez RD.Intraperitoneal radioactive phosphorus (32P) versus observation after negative second-look laparotomy for stage III ovarian carcinoma: a randomized trial of the Gynecologic Oncology Group. Journal of Clinical Oncology 2003;21:2849-55. - PubMed
References to other published versions of this review
Mei 2010
Mei 2013
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

