Optical Coherence Tomography Reveals Distinct Patterns of Retinal Damage in Neuromyelitis Optica and Multiple Sclerosis
- PMID: 23805202
- PMCID: PMC3689687
- DOI: 10.1371/journal.pone.0066151
Optical Coherence Tomography Reveals Distinct Patterns of Retinal Damage in Neuromyelitis Optica and Multiple Sclerosis
Abstract
Background: Neuromyelitis optica (NMO) and relapsing-remitting multiple sclerosis (RRMS) are difficult to differentiate solely on clinical grounds. Optical coherence tomography (OCT) studies investigating retinal changes in both diseases focused primarily on the retinal nerve fiber layer (RNFL) while rare data are available on deeper intra-retinal layers.
Objective: To detect different patterns of intra-retinal layer alterations in patients with NMO spectrum disorders (NMOSD) and RRMS with focus on the influence of a previous optic neuritis (ON).
Methods: We applied spectral-domain OCT in eyes of NMOSD patients and compared them to matched RRMS patients and healthy controls (HC). Semi-automatic intra-retinal layer segmentation was used to quantify intra-retinal layer thicknesses. In a subgroup low contrast visual acuity (LCVA) was assessed.
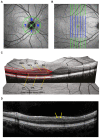
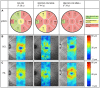
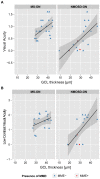
Results: NMOSD-, MS- and HC-groups, each comprising 17 subjects, were included in analysis. RNFL thickness was more severely reduced in NMOSD compared to MS following ON. In MS-ON eyes, RNFL thinning showed a clear temporal preponderance, whereas in NMOSD-ON eyes RNFL was more evenly reduced, resulting in a significantly lower ratio of the nasal versus temporal RNFL thickness. In comparison to HC, ganglion cell layer thickness was stronger reduced in NMOSD-ON than in MS-ON, accompanied by a more severe impairment of LCVA. The inner nuclear layer and the outer retinal layers were thicker in NMOSD-ON patients compared to NMOSD without ON and HC eyes while these differences were primarily driven by microcystic macular edema.
Conclusion: Our study supports previous findings that ON in NMOSD leads to more pronounced retinal thinning and visual function impairment than in RRMS. The different retinal damage patterns in NMOSD versus RRMS support the current notion of distinct pathomechanisms of both conditions. However, OCT is still insufficient to help with the clinically relevant differentiation of both conditions in an individual patient.
Conflict of interest statement
Figures

Similar articles
-
A prospective case-control study comparing optical coherence tomography characteristics in neuromyelitis optica spectrum disorder- optic neuritis and idiopathic optic neuritis.BMC Ophthalmol. 2018 Sep 14;18(1):247. doi: 10.1186/s12886-018-0902-3. BMC Ophthalmol. 2018. PMID: 30217177 Free PMC article.
-
Thickness of macular inner retinal layers and peripapillary retinal nerve fibre layer in neuromyelitis optica spectrum optic neuritis and isolated optic neuritis with one episode.Acta Ophthalmol. 2017 Sep;95(6):583-590. doi: 10.1111/aos.13257. Epub 2016 Oct 24. Acta Ophthalmol. 2017. PMID: 27775238
-
Optical Coherence Tomography in Neuromyelitis Optica spectrum disorder and Multiple Sclerosis: A population-based study.Mult Scler Relat Disord. 2021 Jan;47:102625. doi: 10.1016/j.msard.2020.102625. Epub 2020 Nov 14. Mult Scler Relat Disord. 2021. PMID: 33227631
-
Neuromyelitis optica and multiple sclerosis: Seeing differences through optical coherence tomography.Mult Scler. 2015 May;21(6):678-88. doi: 10.1177/1352458514567216. Epub 2015 Feb 6. Mult Scler. 2015. PMID: 25662342 Free PMC article. Review.
-
A comparative study of alteration in retinal layer segmentation alteration by SD-OCT in neuromyelitis optica spectrum disorders: A systematic review and meta-analysis.Adv Ophthalmol Pract Res. 2021 Oct 5;1(1):100007. doi: 10.1016/j.aopr.2021.100007. eCollection 2021 Nov. Adv Ophthalmol Pract Res. 2021. PMID: 37846392 Free PMC article. Review.
Cited by
-
MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients.J Neuroinflammation. 2016 Nov 1;13(1):282. doi: 10.1186/s12974-016-0720-6. J Neuroinflammation. 2016. PMID: 27802824 Free PMC article.
-
Association of Visual Impairment in Neuromyelitis Optica Spectrum Disorder With Visual Network Reorganization.JAMA Neurol. 2018 Mar 1;75(3):296-303. doi: 10.1001/jamaneurol.2017.3890. JAMA Neurol. 2018. PMID: 29297041 Free PMC article.
-
Ultrahigh field MRI in clinical neuroimmunology: a potential contribution to improved diagnostics and personalised disease management.EPMA J. 2015 Aug 27;6(1):16. doi: 10.1186/s13167-015-0038-y. eCollection 2015. EPMA J. 2015. PMID: 26312125 Free PMC article. Review.
-
Reliability of Intra-Retinal Layer Thickness Estimates.PLoS One. 2015 Sep 8;10(9):e0137316. doi: 10.1371/journal.pone.0137316. eCollection 2015. PLoS One. 2015. PMID: 26349053 Free PMC article.
-
3D-DIR for early differential diagnostic and prognostic evaluation of NMO.Exp Ther Med. 2016 Sep;12(3):1464-1468. doi: 10.3892/etm.2016.3474. Epub 2016 Jun 23. Exp Ther Med. 2016. PMID: 27588068 Free PMC article.
References
-
- Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG (1999) The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 53: 1107–1114. - PubMed
-
- Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, et al. (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364: 2106–2112. - PubMed
-
- Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG (2007) The spectrum of neuromyelitis optica. Lancet Neurol 6: 805–815. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

