The impact of pathogenic mitochondrial DNA mutations on substantia nigra neurons
- PMID: 23804100
- PMCID: PMC6618501
- DOI: 10.1523/JNEUROSCI.3525-12.2013
The impact of pathogenic mitochondrial DNA mutations on substantia nigra neurons
Abstract
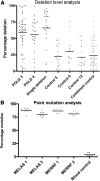
Mitochondrial defects within substantia nigra (SN) neurons are implicated in the pathogenesis of Parkinson's disease. SN neurons show increased mitochondrial defects, mitochondrial DNA deletion levels, and susceptibility to such dysfunction, although the role of mitochondria in neuronal degeneration remains uncertain. In this study, we addressed this important question by exploring changes within the mitochondria of SN neurons from patients with primary mitochondrial diseases to determine whether mitochondrial dysfunction leads directly to neuronal cell loss. We counted the pigmented neurons and quantified mitochondrial respiratory activity, deficiencies in mitochondrial proteins, and the percentage of pathogenic mutations in single neurons. We found evidence of defects of both complex I and complex IV of the respiratory chain in all patients. We found that marked neuronal cell loss was only observed in a few patients with mitochondrial disease and that all these patients had mutations in polymerase gamma (POLG), which leads to the formation of multiple mitochondrial DNA deletions over time, similar to aging and Parkinson's disease. Interestingly, we detected α-synuclein pathology in two mitochondrial patients with POLG mutations. Our observations highlight the complex relationship between mitochondrial dysfunction and the susceptibility of SN neurons to degeneration and α-synuclein pathology. Our finding that the loss of SN neurons was only severe in patients with POLG mutations suggests that acquired mitochondrial defects may be less well tolerated by SN neurons than by inherited ones.
Figures
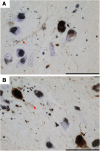
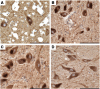
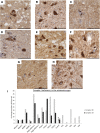

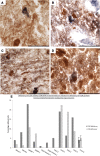
Comment in
-
Are substantia nigra neurons subject to mitochondrial dysfunction in early life more able to adapt?Mov Disord. 2013 Oct;28(12):1638. doi: 10.1002/mds.25651. Epub 2013 Oct 1. Mov Disord. 2013. PMID: 24114880 No abstract available.
Similar articles
-
Severe nigrostriatal degeneration without clinical parkinsonism in patients with polymerase gamma mutations.Brain. 2013 Aug;136(Pt 8):2393-404. doi: 10.1093/brain/awt103. Epub 2013 Apr 26. Brain. 2013. PMID: 23625061
-
Relationship between mitochondria and α-synuclein: a study of single substantia nigra neurons.Arch Neurol. 2012 Mar;69(3):385-93. doi: 10.1001/archneurol.2011.2675. Arch Neurol. 2012. PMID: 22410447
-
Accumulation of mitochondrial DNA deletions within dopaminergic neurons triggers neuroprotective mechanisms.Brain. 2013 Aug;136(Pt 8):2369-78. doi: 10.1093/brain/awt196. Brain. 2013. PMID: 23884809
-
Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse.Free Radic Biol Med. 2013 Sep;62:186-201. doi: 10.1016/j.freeradbiomed.2013.05.042. Epub 2013 Jun 3. Free Radic Biol Med. 2013. PMID: 23743292 Review.
-
[Lewy body formation in Parkinson's disease: neurodegeneration or neuroprotection?].Rinsho Shinkeigaku. 2008 Nov;48(11):981-3. doi: 10.5692/clinicalneurol.48.981. Rinsho Shinkeigaku. 2008. PMID: 19198138 Review. Japanese.
Cited by
-
Parkinson's disease as a result of aging.Aging Cell. 2015 Jun;14(3):293-308. doi: 10.1111/acel.12312. Epub 2015 Feb 9. Aging Cell. 2015. PMID: 25677794 Free PMC article. Review.
-
Ageing and Parkinson's disease: why is advancing age the biggest risk factor?Ageing Res Rev. 2014 Mar;14(100):19-30. doi: 10.1016/j.arr.2014.01.004. Epub 2014 Feb 3. Ageing Res Rev. 2014. PMID: 24503004 Free PMC article. Review.
-
Extensive respiratory chain defects in inhibitory interneurones in patients with mitochondrial disease.Neuropathol Appl Neurobiol. 2016 Feb;42(2):180-93. doi: 10.1111/nan.12238. Epub 2015 May 30. Neuropathol Appl Neurobiol. 2016. PMID: 25786813 Free PMC article.
-
Mitochondrial Dysfunction in Parkinson's Disease-Cause or Consequence?Biology (Basel). 2019 May 11;8(2):38. doi: 10.3390/biology8020038. Biology (Basel). 2019. PMID: 31083583 Free PMC article. Review.
-
The mitochondrial transcription factor TFAM in neurodegeneration: emerging evidence and mechanisms.FEBS Lett. 2018 Mar;592(5):793-811. doi: 10.1002/1873-3468.12989. Epub 2018 Feb 15. FEBS Lett. 2018. PMID: 29364506 Free PMC article. Review.
References
-
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. - DOI - PubMed
-
- Betts J, Jaros E, Perry RH, Schaefer AM, Taylor RW, Abdel-All Z, Lightowlers RN, Turnbull DM. Molecular neuropathology of MELAS: level of heteroplasmy in individual neurones and evidence of extensive vascular involvement. Neuropathol Appl Neurobiol. 2006;32:359–373. doi: 10.1111/j.1365-2990.2006.00731.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
