Pulmonary arterial hypertension: basis of sex differences in incidence and treatment response
- PMID: 23802760
- PMCID: PMC3969073
- DOI: 10.1111/bph.12281
Pulmonary arterial hypertension: basis of sex differences in incidence and treatment response
Abstract
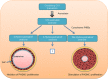
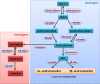
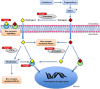
Pulmonary arterial hypertension (PAH) is a complex disease characterized by elevated pulmonary arterial pressure, pulmonary vascular remodelling and occlusive pulmonary vascular lesions, leading to right heart failure. Evidence from recent epidemiological studies suggests the influence of gender on the development of PAH with an approximate female to male ratio of 4:1, depending on the underlying disease pathology. Overall, the therapeutic strategy for PAH remains suboptimal with poor survival rates observed in both genders. Endogenous sex hormones, in particular 17β oestradiol and its metabolites, have been implicated in the development of the disease; however, the influence of sex hormones on the underlying pathobiology remains controversial. Further understanding of the influence of sex hormones on the normal and diseased pulmonary circulation will be critical to our understanding the pathology of PAH and future therapeutic strategies. In this review, we will discuss the influence of sex hormones on the development of PAH and address recent controversies.
Keywords: oestrogen; pulmonary arterial hypertension; serotonin; sex hormones; testosterone; vascular remodelling.
© 2013 The British Pharmacological Society.
Figures
Similar articles
-
Progress in solving the sex hormone paradox in pulmonary hypertension.Am J Physiol Lung Cell Mol Physiol. 2014 Jul 1;307(1):L7-26. doi: 10.1152/ajplung.00337.2013. Epub 2014 May 9. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24816487 Review.
-
The Role of Sex in the Pathophysiology of Pulmonary Hypertension.Adv Exp Med Biol. 2018;1065:511-528. doi: 10.1007/978-3-319-77932-4_31. Adv Exp Med Biol. 2018. PMID: 30051404 Review.
-
Sex differences in the pulmonary circulation: implications for pulmonary hypertension.Am J Physiol Heart Circ Physiol. 2014 May;306(9):H1253-64. doi: 10.1152/ajpheart.00857.2013. Epub 2014 Mar 7. Am J Physiol Heart Circ Physiol. 2014. PMID: 24610923 Free PMC article. Review.
-
The serotonin transporter, gender, and 17β oestradiol in the development of pulmonary arterial hypertension.Cardiovasc Res. 2011 May 1;90(2):373-82. doi: 10.1093/cvr/cvq408. Epub 2010 Dec 22. Cardiovasc Res. 2011. PMID: 21177701
-
[Female gender and pulmonary arterial hypertension: a complex relationship].G Ital Cardiol (Rome). 2012 Jun;13(6):448-60. doi: 10.1714/1073.11764. G Ital Cardiol (Rome). 2012. PMID: 22622125 Italian.
Cited by
-
Estrogen-mediated mechanisms in hypertension and other cardiovascular diseases.J Hum Hypertens. 2023 Aug;37(8):609-618. doi: 10.1038/s41371-022-00771-0. Epub 2022 Nov 1. J Hum Hypertens. 2023. PMID: 36319856 Free PMC article. Review.
-
ET-1 as a Sex-Specific Mechanism Impacting Age-Related Changes in Vascular Function.Front Aging. 2021 Aug 31;2:727416. doi: 10.3389/fragi.2021.727416. eCollection 2021. Front Aging. 2021. PMID: 35822003 Free PMC article. Review.
-
Pulmonary Hypertension in Women.Methodist Debakey Cardiovasc J. 2024 Mar 14;20(2):70-80. doi: 10.14797/mdcvj.1308. eCollection 2024. Methodist Debakey Cardiovasc J. 2024. PMID: 38495664 Free PMC article. Review.
-
'Tired, afraid, breathless … .' An international survey of the exercise experience for people living with pulmonary hypertension.Pulm Circ. 2020 Nov 16;10(4):2045894020968023. doi: 10.1177/2045894020968023. eCollection 2020 Oct-Dec. Pulm Circ. 2020. PMID: 33240490 Free PMC article.
-
Newer insights into the pathobiological and pharmacological basis of the sex disparity in patients with pulmonary arterial hypertension.Am J Physiol Lung Cell Mol Physiol. 2021 Jun 1;320(6):L1025-L1037. doi: 10.1152/ajplung.00559.2020. Epub 2021 Mar 10. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33719549 Free PMC article. Review.
References
-
- Badawi AF, Cavalieri EL, Rogan EG. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism. 2001;50:1001–1003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

