Histone deacetylase 10 promotes autophagy-mediated cell survival
- PMID: 23801752
- PMCID: PMC3710791
- DOI: 10.1073/pnas.1300113110
Histone deacetylase 10 promotes autophagy-mediated cell survival
Abstract
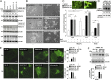
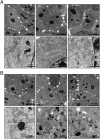
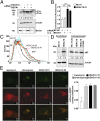
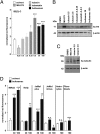
Tumor cells activate autophagy in response to chemotherapy-induced DNA damage as a survival program to cope with metabolic stress. Here, we provide in vitro and in vivo evidence that histone deacetylase (HDAC)10 promotes autophagy-mediated survival in neuroblastoma cells. We show that both knockdown and inhibition of HDAC10 effectively disrupted autophagy associated with sensitization to cytotoxic drug treatment in a panel of highly malignant V-MYC myelocytomatosis viral-related oncogene, neuroblastoma derived-amplified neuroblastoma cell lines, in contrast to nontransformed cells. HDAC10 depletion in neuroblastoma cells interrupted autophagic flux and induced accumulation of autophagosomes, lysosomes, and a prominent substrate of the autophagic degradation pathway, p62/sequestosome 1. Enforced HDAC10 expression protected neuroblastoma cells against doxorubicin treatment through interaction with heat shock protein 70 family proteins, causing their deacetylation. Conversely, heat shock protein 70/heat shock cognate 70 was acetylated in HDAC10-depleted cells. HDAC10 expression levels in high-risk neuroblastomas correlated with autophagy in gene-set analysis and predicted treatment success in patients with advanced stage 4 neuroblastomas. Our results demonstrate that HDAC10 protects cancer cells from cytotoxic agents by mediating autophagy and identify this HDAC isozyme as a druggable regulator of advanced-stage tumor cell survival. Moreover, these results propose a promising way to considerably improve treatment response in the neuroblastoma patient subgroup with the poorest outcome.
Keywords: HDAC inhibitor; childhood tumors; drug resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Histone deacetylase 10-promoted autophagy as a druggable point of interference to improve the treatment response of advanced neuroblastomas.Autophagy. 2013 Dec;9(12):2163-5. doi: 10.4161/auto.26450. Epub 2013 Oct 8. Autophagy. 2013. PMID: 24145760
-
Dual role of HDAC10 in lysosomal exocytosis and DNA repair promotes neuroblastoma chemoresistance.Sci Rep. 2018 Jul 3;8(1):10039. doi: 10.1038/s41598-018-28265-5. Sci Rep. 2018. PMID: 29968769 Free PMC article.
-
Identification of histone deacetylase 10 (HDAC10) inhibitors that modulate autophagy in transformed cells.Eur J Med Chem. 2022 Apr 15;234:114272. doi: 10.1016/j.ejmech.2022.114272. Epub 2022 Mar 11. Eur J Med Chem. 2022. PMID: 35306288 Free PMC article.
-
Polyamine Deacetylase Structure and Catalysis: Prokaryotic Acetylpolyamine Amidohydrolase and Eukaryotic HDAC10.Biochemistry. 2018 Jun 5;57(22):3105-3114. doi: 10.1021/acs.biochem.8b00079. Epub 2018 Mar 21. Biochemistry. 2018. PMID: 29533602 Free PMC article. Review.
-
Targeting histone deacetylases in neuroblastoma.Curr Pharm Des. 2009;15(4):436-47. doi: 10.2174/138161209787315774. Curr Pharm Des. 2009. PMID: 19199971 Review.
Cited by
-
Histone deacetylase 10, a potential epigenetic target for therapy.Biosci Rep. 2021 Jun 25;41(6):BSR20210462. doi: 10.1042/BSR20210462. Biosci Rep. 2021. PMID: 33997894 Free PMC article. Review.
-
Structural Basis for the Selective Inhibition of HDAC10, the Cytosolic Polyamine Deacetylase.ACS Chem Biol. 2020 Aug 21;15(8):2154-2163. doi: 10.1021/acschembio.0c00362. Epub 2020 Jul 23. ACS Chem Biol. 2020. PMID: 32659072 Free PMC article.
-
Characterization of the Role of Host Cellular Factor Histone Deacetylase 10 during HIV-1 Replication.Viruses. 2019 Dec 26;12(1):28. doi: 10.3390/v12010028. Viruses. 2019. PMID: 31888084 Free PMC article.
-
Sirtuin 6 overexpression relieves sepsis-induced acute kidney injury by promoting autophagy.Cell Cycle. 2019 Feb;18(4):425-436. doi: 10.1080/15384101.2019.1568746. Epub 2019 Jan 30. Cell Cycle. 2019. PMID: 30700227 Free PMC article.
-
Acetylation of STX17 (syntaxin 17) controls autophagosome maturation.Autophagy. 2021 May;17(5):1157-1169. doi: 10.1080/15548627.2020.1752471. Epub 2020 Apr 15. Autophagy. 2021. PMID: 32264736 Free PMC article.
References
-
- Pan Y, et al. Targeting autophagy augments in vitro and in vivo antimyeloma activity of DNA-damaging chemotherapy. Clin Cancer Res. 2011;17(10):3248–3258. - PubMed
-
- Klionsky DJ, Cuervo AM, Seglen PO. Methods for monitoring autophagy from yeast to human. Autophagy. 2007;3(3):181–206. - PubMed
-
- Dice JF. Chaperone-mediated autophagy. Autophagy. 2007;3(4):295–299. - PubMed
-
- Agarraberes FA, Dice JF. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci. 2001;114(Pt 13):2491–2499. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

