Live-virus exposure of vaccine-protected macaques alters the anti-HIV-1 antibody repertoire in the absence of viremia
- PMID: 23800339
- PMCID: PMC3695773
- DOI: 10.1186/1742-4690-10-63
Live-virus exposure of vaccine-protected macaques alters the anti-HIV-1 antibody repertoire in the absence of viremia
Abstract
Background: We addressed the question whether live-virus challenges could alter vaccine-induced antibody (Ab) responses in vaccinated rhesus macaques (RMs) that completely resisted repeated exposures to R5-tropic simian-human immunodeficiency viruses encoding heterologous HIV clade C envelopes (SHIV-Cs).
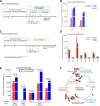
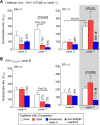
Results: We examined the Ab responses in aviremic RMs that had been immunized with a multi-component protein vaccine (multimeric HIV-1 gp160, HIV-1 Tat and SIV Gag-Pol particles) and compared anti-Env plasma Ab titers before and after repeated live-virus exposures. Although no viremia was ever detected in these animals, they showed significant increases in anti-gp140 Ab titers after they had encountered live SHIVs. When we investigated the dynamics of anti-Env Ab titers during the immunization and challenge phases further, we detected the expected, vaccine-induced increases of Ab responses about two weeks after the last protein immunization. Remarkably, these titers kept rising during the repeated virus challenges, although no viremia resulted. In contrast, in vaccinated RMs that were not exposed to virus, anti-gp140 Ab titers declined after the peak seen two weeks after the last immunization. These data suggest boosting of pre-existing, vaccine-induced Ab responses as a consequence of repeated live-virus exposures. Next, we screened polyclonal plasma samples from two of the completely protected vaccinees by peptide phage display and designed a strategy that selects for recombinant phages recognized only by Abs present after - but not before - any SHIV challenge. With this "subtractive biopanning" approach, we isolated V3 mimotopes that were only recognized after the animals had been exposed to live virus. By detailed epitope mapping of such anti-V3 Ab responses, we showed that the challenges not only boosted pre-existing binding and neutralizing Ab titers, but also induced Abs targeting neo-antigens presented by the heterologous challenge virus.
Conclusions: Anti-Env Ab responses induced by recombinant protein vaccination were altered by the multiple, live SHIV challenges in vaccinees that had no detectable viral loads. These data may have implications for the interpretation of "vaccine only" responses in clinical vaccine trials.
Figures

Similar articles
-
Novel biopanning strategy to identify epitopes associated with vaccine protection.J Virol. 2013 Apr;87(8):4403-16. doi: 10.1128/JVI.02888-12. Epub 2013 Feb 6. J Virol. 2013. PMID: 23388727 Free PMC article.
-
Immunization with wild-type or CD4-binding-defective HIV-1 Env trimers reduces viremia equivalently following heterologous challenge with simian-human immunodeficiency virus.J Virol. 2010 Sep;84(18):9086-95. doi: 10.1128/JVI.01015-10. Epub 2010 Jul 7. J Virol. 2010. PMID: 20610729 Free PMC article.
-
Combination Adenovirus and Protein Vaccines Prevent Infection or Reduce Viral Burden after Heterologous Clade C Simian-Human Immunodeficiency Virus Mucosal Challenge.J Virol. 2018 Jan 2;92(2):e01092-17. doi: 10.1128/JVI.01092-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29093095 Free PMC article.
-
Vaccine-induced V1V2-specific antibodies control and or protect against infection with HIV, SIV and SHIV.Curr Opin HIV AIDS. 2019 Jul;14(4):309-317. doi: 10.1097/COH.0000000000000551. Curr Opin HIV AIDS. 2019. PMID: 30994501 Free PMC article. Review.
-
Advances in simian--human immunodeficiency viruses for nonhuman primate studies of HIV prevention and cure.Curr Opin HIV AIDS. 2020 Sep;15(5):275-281. doi: 10.1097/COH.0000000000000645. Curr Opin HIV AIDS. 2020. PMID: 32769631 Review.
Cited by
-
Multimodality vaccination against clade C SHIV: partial protection against mucosal challenges with a heterologous tier 2 virus.Vaccine. 2014 Nov 12;32(48):6527-36. doi: 10.1016/j.vaccine.2014.08.065. Epub 2014 Sep 20. Vaccine. 2014. PMID: 25245933 Free PMC article.
References
-
- Progress report 2011: Global HIV/AIDS response (UNAIDS) [ http://www.who.int/hiv/pub/progress_report2011/en/index.html]
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

