Incidence and risk of treatment-related mortality with mTOR inhibitors everolimus and temsirolimus in cancer patients: a meta-analysis
- PMID: 23785409
- PMCID: PMC3681778
- DOI: 10.1371/journal.pone.0065166
Incidence and risk of treatment-related mortality with mTOR inhibitors everolimus and temsirolimus in cancer patients: a meta-analysis
Abstract
Background: Two novel mammalian targets of rapamycin (mTOR) inhibitors everolimus and temsirolimus are now approved by regulatory agencies and have been widely investigated among various types of solid tumors, but the risk of fatal adverse events (FAEs) with these drugs is not well defined.
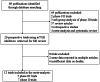
Methods: We searched PubMed, EMBASE, and Cochrane library databases for relevant trials. Eligible studies included prospective phase II and III trials evaluating everolimus and temsirolimus in patients with all malignancies and data on FAEs were available. Statistical analyses were conducted to calculate the summary incidence, RRs and 95% confidence intervals (CIs) by using either random effects or fixed effect models according to the heterogeneity of the included studies.
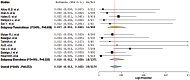
Results: A total of 3322 patients with various advanced solid tumors from 12 trials were included. The overall incidence of mTOR inhibitors associated FAEs was 1.8% (95%CI: 1.3-2.5%), and the incidences of everolimus related FAEs were comparable to that of temsirolimus (1.7% versus 1.8%). Compared with the controls, the use of mTOR inhibitors was associated with an increased risk of FAEs, with a RR of 3.24 (95%CI: 1.21-8.67, p = 0.019). On subgroup analysis, a non-statistically significant increase in the risk of FAEs was found according to different mTOR inhibitors, tumor types or controlled therapy. No evidence of publication bias was observed.
Conclusion: With the present evidence, the use of mTOR inhibitors seems to increase the risk of FAEs in patients with advanced solid tumors. More high quality trials are still needed to investigate this association.
Conflict of interest statement
Figures

Similar articles
-
Hematologic toxicities associated with mTOR inhibitors temsirolimus and everolimus in cancer patients: a systematic review and meta-analysis.Curr Med Res Opin. 2014 Jan;30(1):67-74. doi: 10.1185/03007995.2013.844116. Epub 2013 Oct 4. Curr Med Res Opin. 2014. PMID: 24028709 Review.
-
Incidence and risk of treatment-related mortality in cancer patients treated with the mammalian target of rapamycin inhibitors.Ann Oncol. 2013 Aug;24(8):2092-7. doi: 10.1093/annonc/mdt155. Epub 2013 May 8. Ann Oncol. 2013. PMID: 23658373
-
Treatment-related fatigue with everolimus and temsirolimus in patients with cancer-a meta-analysis of clinical trials.Tumour Biol. 2015 Feb;36(2):643-54. doi: 10.1007/s13277-014-2669-3. Epub 2014 Oct 4. Tumour Biol. 2015. PMID: 25281033
-
Attributable Risk of Infection to mTOR Inhibitors Everolimus and Temsirolimus in the Treatment of Cancer.Cancer Invest. 2016 Nov 25;34(10):521-530. doi: 10.1080/07357907.2016.1242009. Epub 2016 Oct 28. Cancer Invest. 2016. PMID: 27791402 Review.
-
Incidence and risk of high-grade stomatitis with mTOR inhibitors in cancer patients.Cancer Invest. 2015 Mar;33(3):70-7. doi: 10.3109/07357907.2014.1001893. Epub 2015 Jan 30. Cancer Invest. 2015. PMID: 25635371
Cited by
-
Sulindac (K-80003) with nab-paclitaxel and gemcitabine overcomes drug-resistant pancreatic cancer.Mol Cancer. 2024 Sep 30;23(1):215. doi: 10.1186/s12943-024-02128-2. Mol Cancer. 2024. PMID: 39350121 Free PMC article.
-
Complex Interplay between DNA Damage and Autophagy in Disease and Therapy.Biomolecules. 2024 Jul 29;14(8):922. doi: 10.3390/biom14080922. Biomolecules. 2024. PMID: 39199310 Free PMC article. Review.
-
CPT-11 mitigates autoimmune diseases by suppressing effector T cells without affecting long-term anti-tumor immunity.Cell Death Discov. 2024 May 4;10(1):218. doi: 10.1038/s41420-024-01983-8. Cell Death Discov. 2024. PMID: 38704362 Free PMC article.
-
Perturbed autophagy intervenes systemic lupus erythematosus by active ingredients of traditional Chinese medicine.Front Pharmacol. 2023 Jan 17;13:1053602. doi: 10.3389/fphar.2022.1053602. eCollection 2022. Front Pharmacol. 2023. PMID: 36733375 Free PMC article. Review.
-
Protocadherin gamma C3: a new player in regulating vascular barrier function.Neural Regen Res. 2023 Jan;18(1):68-73. doi: 10.4103/1673-5374.343896. Neural Regen Res. 2023. PMID: 35799511 Free PMC article. Review.
References
-
- Fasolo A, Sessa C (2012) Targeting mTOR pathways in human malignancies. Curr Pharm Des 18: 2766–2777. - PubMed
-
- Brugarolas J (2007) Renal-cell carcinoma – molecular pathways and therapies. N Engl J Med 356: 185–187. - PubMed
-
- Sheppard K, Kinross KM, Solomon B, Pearson RB, Phillips WA (2012) Targeting PI3 kinase/AKT/mTOR signaling in cancer. Crit Rev Oncog 17: 69–95. - PubMed
-
- Faivre S, Kroemer G, Raymond E (2006) Current development of mTOR inhibitors as anticancer agents. Nature Reviews Drug Discovery 5: 671–688. - PubMed
-
- Gnant M (2012) Overcoming endocrine resistance in breast cancer: importance of mTOR inhibition. Expert Rev Anticancer Ther 12: 1579–1589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

