Adipokines mediate inflammation and insulin resistance
- PMID: 23781214
- PMCID: PMC3679475
- DOI: 10.3389/fendo.2013.00071
Adipokines mediate inflammation and insulin resistance
Abstract
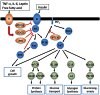
For many years, adipose tissue was considered as an inert energy storage organ that accumulates and stores triacylglycerols during energy excess and releases fatty acids in times of systemic energy need. However, over the last two decades adipose tissue depots have been established as highly active endocrine and metabolically important organs that modulate energy expenditure and glucose homeostasis. In rodents, brown adipose tissue plays an essential role in non-shivering thermogenesis and in energy dissipation that can serve to protect against diet-induced obesity. White adipose tissue collectively referred too as either subcutaneous or visceral adipose tissue is responsible for the secretion of an array of signaling molecules, termed adipokines. These adipokines function as classic circulating hormones to communicate with other organs including brain, liver, muscle, the immune system, and adipose tissue itself. The dysregulation of adipokines has been implicated in obesity, type 2 diabetes, and cardiovascular disease. Recently, inflammatory responses in adipose tissue have been shown as a major mechanism to induce peripheral tissue insulin resistance. Although leptin and adiponectin regulate feeding behavior and energy expenditure, these adipokines are also involved in the regulation of inflammatory responses. Adipose tissue secretes various pro- and anti-inflammatory adipokines to modulate inflammation and insulin resistance. In obese humans and rodent models, the expression of pro-inflammatory adipokines is enhanced to induce insulin resistance. Collectively, these findings have suggested that obesity-induced insulin resistance may result, at least in part, from an imbalance in the expression of pro- and anti-inflammatory adipokines. Thus we will review the recent progress regarding the physiological and molecular functions of adipokines in the obesity-induced inflammation and insulin resistance with perspectives on future directions.
Keywords: adipocyte; adipokine; inflammation; insulin; macrophages and metabolism.
Figures

Similar articles
-
Recent advances in the relationship between obesity, inflammation, and insulin resistance.Eur Cytokine Netw. 2006 Mar;17(1):4-12. Eur Cytokine Netw. 2006. PMID: 16613757 Review.
-
Nrg4 promotes fuel oxidation and a healthy adipokine profile to ameliorate diet-induced metabolic disorders.Mol Metab. 2017 Jun 21;6(8):863-872. doi: 10.1016/j.molmet.2017.03.016. eCollection 2017 Aug. Mol Metab. 2017. PMID: 28752050 Free PMC article.
-
Adipose tissue as an endocrine organ.Mol Cell Endocrinol. 2010 Mar 25;316(2):129-39. doi: 10.1016/j.mce.2009.08.018. Epub 2009 Aug 31. Mol Cell Endocrinol. 2010. PMID: 19723556 Review.
-
Secretory function of adipose tissue.Pol J Vet Sci. 2016;19(2):441-6. doi: 10.1515/pjvs-2016-0056. Pol J Vet Sci. 2016. PMID: 27487522 Review.
-
Circulating adipokines and mRNA expression in adipose tissue and the placenta in women with gestational diabetes mellitus.Peptides. 2018 Mar;101:157-166. doi: 10.1016/j.peptides.2018.01.005. Epub 2018 Jan 11. Peptides. 2018. PMID: 29337272 Clinical Trial.
Cited by
-
The Cardiometabolic Risk in Women with Polycystic Ovarian Syndrome (PCOS): From Pathophysiology to Diagnosis and Treatment.Medicina (Kaunas). 2024 Oct 10;60(10):1656. doi: 10.3390/medicina60101656. Medicina (Kaunas). 2024. PMID: 39459443 Free PMC article. Review.
-
Stimulation of soluble guanylyl cyclase protects against obesity by recruiting brown adipose tissue.Nat Commun. 2015 May 26;6:7235. doi: 10.1038/ncomms8235. Nat Commun. 2015. PMID: 26011238 Free PMC article.
-
Diet-induced obesity causes insulin resistance in mouse brown adipose tissue.Obesity (Silver Spring). 2015 Sep;23(9):1765-70. doi: 10.1002/oby.21134. Epub 2015 Aug 3. Obesity (Silver Spring). 2015. PMID: 26242777 Free PMC article.
-
Association between pre-diagnostic circulating adipokines and colorectal cancer and adenoma in the CLUE II cohort.Cancer Causes Control. 2021 Aug;32(8):871-881. doi: 10.1007/s10552-021-01441-1. Epub 2021 May 17. Cancer Causes Control. 2021. PMID: 33999316 Free PMC article.
-
Adiponectin and pro-inflammatory cytokines are modulated in Vietnamese patients with type 2 diabetes mellitus.J Diabetes Investig. 2017 May;8(3):295-305. doi: 10.1111/jdi.12579. Epub 2016 Oct 30. J Diabetes Investig. 2017. PMID: 27684566 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

