Orchestration of CD4 T cell epitope preferences after multipeptide immunization
- PMID: 23772029
- PMCID: PMC3742317
- DOI: 10.4049/jimmunol.1300312
Orchestration of CD4 T cell epitope preferences after multipeptide immunization
Abstract
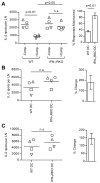
A detailed understanding of the molecular and cellular mechanisms that underlie epitope preferences in T cell priming is important for vaccines designed to elicit a broad T cell response. Protein vaccinations generally elicit CD4 T cell responses that are skewed toward a small fraction of epitopes, a phenomenon known as immunodominance. This characteristic of T cell responses, which limits the diversity of CD4 T cell recognition, is generally attributed to intracellular Ag processing. However, we recently discovered that immunodominance hierarchies persist even after vaccination with synthetic peptides. In this study, we probed the regulatory mechanisms that cause diminished CD4 T cell responses to subdominant peptides after such multipeptide immunization in mice. We have found that the delivery of subdominant and dominant epitopes on separate dendritic cells rescues expansion of less favored CD4 T cells. Furthermore, through the use of genetic models and inhibitors, we have found that selective losses in CD4 T cell responses are mediated by an IFN-γ-induced pathway, involving IDO, and that regulatory T cell activities may also regulate preferences in CD4 T cell specificity. We propose that after multipeptide immunization, the expansion and differentiation of dominant T cells initiate complex regulatory events that determine the final peptide specificity of the elicited CD4 T cell response.
Figures

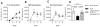

Similar articles
-
The control of the specificity of CD4 T cell responses: thresholds, breakpoints, and ceilings.Front Immunol. 2013 Oct 23;4:340. doi: 10.3389/fimmu.2013.00340. Front Immunol. 2013. PMID: 24167504 Free PMC article. Review.
-
Immunodominance in virus-induced CD8(+) T-cell responses is dramatically modified by DNA immunization and is regulated by gamma interferon.J Virol. 2002 May;76(9):4251-9. doi: 10.1128/jvi.76.9.4251-4259.2002. J Virol. 2002. PMID: 11932390 Free PMC article.
-
Quality and vaccine efficacy of CD4+ T cell responses directed to dominant and subdominant epitopes in ESAT-6 from Mycobacterium tuberculosis.J Immunol. 2009 Aug 15;183(4):2659-68. doi: 10.4049/jimmunol.0900947. Epub 2009 Jul 20. J Immunol. 2009. PMID: 19620314
-
Inefficient cross-presentation limits the CD8+ T cell response to a subdominant tumor antigen epitope.J Immunol. 2005 Jul 15;175(2):700-12. doi: 10.4049/jimmunol.175.2.700. J Immunol. 2005. PMID: 16002665
-
A replication competent adenovirus 5 host range mutant-simian immunodeficiency virus (SIV) recombinant priming/subunit protein boosting vaccine regimen induces broad, persistent SIV-specific cellular immunity to dominant and subdominant epitopes in Mamu-A*01 rhesus macaques.J Immunol. 2003 Apr 15;170(8):4281-9. doi: 10.4049/jimmunol.170.8.4281. J Immunol. 2003. PMID: 12682263
Cited by
-
The control of the specificity of CD4 T cell responses: thresholds, breakpoints, and ceilings.Front Immunol. 2013 Oct 23;4:340. doi: 10.3389/fimmu.2013.00340. Front Immunol. 2013. PMID: 24167504 Free PMC article. Review.
-
CD4 T cells in protection from influenza virus: Viral antigen specificity and functional potential.Immunol Rev. 2018 Jul;284(1):91-105. doi: 10.1111/imr.12662. Immunol Rev. 2018. PMID: 29944766 Free PMC article. Review.
-
Discovery of novel cross-protective Rickettsia prowazekii T-cell antigens using a combined reverse vaccinology and in vivo screening approach.Vaccine. 2014 Sep 3;32(39):4968-76. doi: 10.1016/j.vaccine.2014.06.089. Epub 2014 Jul 7. Vaccine. 2014. PMID: 25010827 Free PMC article.
-
Randomized peptide assemblies for enhancing immune responses to nanomaterials.Biomaterials. 2021 Jun;273:120825. doi: 10.1016/j.biomaterials.2021.120825. Epub 2021 Apr 15. Biomaterials. 2021. PMID: 33901731 Free PMC article.
-
Heterologous viral protein interactions within licensed seasonal influenza virus vaccines.NPJ Vaccines. 2020 Jan 10;5(1):3. doi: 10.1038/s41541-019-0153-1. eCollection 2020. NPJ Vaccines. 2020. PMID: 31934357 Free PMC article.
References
-
- Scharnagl NC, Klade CS. Experimental discovery of T-cell epitopes: combining the best of classical and contemporary approaches. Expert Rev Vaccines. 2007;6:605–615. - PubMed
-
- Dudek NL, Perlmutter P, Aguilar MI, Croft NP, Purcell AW. Epitope discovery and their use in peptide based vaccines. Curr Pharm Des. 2010;16:3149–3157. - PubMed
-
- Sette A, Fikes J. Epitope-based vaccines: an update on epitope identification, vaccine design and delivery. Current Opinion in Immunology. 2003;15:461–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

