Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling
- PMID: 23766441
- PMCID: PMC4083824
- DOI: 10.1136/gutjnl-2012-304241
Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling
Abstract
Objective: Claudin-1 expression is increased and dysregulated in colorectal cancer and causally associates with the dedifferentiation of colonic epithelial cells, cancer progression and metastasis. Here, we have sought to determine the role claudin-1 plays in the regulation of intestinal epithelial homeostasis.
Design: We have used a novel villin-claudin-1 transgenic (Cl-1Tg) mouse as model (with intestinal claudin-1 overexpression). The effect of claudin-1 expression upon colonic epithelial differentiation, lineage commitment and Notch-signalling was determined using immunohistochemical, immunoblot and real-time PCR analysis. The frequently used mouse model of dextran sodium sulfate (DSS)-colitis was used to model inflammation, injury and repair.
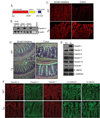
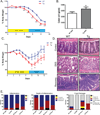
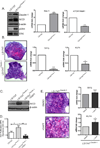
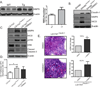
Results: In Cl-1Tg mice, normal colonocyte differentiation programme was disrupted and goblet cell number and mucin-2 (muc-2) expressions were significantly downregulated while Notch- and ERK1/2-signalling were upregulated, compared with the wild type-littermates. Cl-1Tg mice were also susceptible to colonic inflammation and demonstrated impaired recovery and hyperproliferation following the DSS-colitis. Our data further show that claudin-1 regulates Notch-signalling through the regulation of matrix metalloproteinase-9 (MMP-9) and p-ERK signalling to regulate proliferation and differentiation.
Conclusions: Claudin-1 helps regulate intestinal epithelial homeostasis through the regulation of Notch-signalling. An upregulated claudin-1 expression induces MMP-9 and p-ERK signalling to activate Notch-signalling, which in turn inhibits the goblet cell differentiation. Decreased goblet cell number decreases muc-2 expression and thus enhances susceptibility to mucosal inflammation. Claudin-1 expression also induces colonic epithelial proliferation in a Notch-dependent manner. Our findings may help understand the role of claudin-1 in the regulation of inflammatory bowel diseases and CRC.
Keywords: Differentiation; Inflammation; Mucin-2; Notch.
Figures




Similar articles
-
Upregulated claudin-1 expression promotes colitis-associated cancer by promoting β-catenin phosphorylation and activation in Notch/p-AKT-dependent manner.Oncogene. 2019 Jun;38(26):5321-5337. doi: 10.1038/s41388-019-0795-5. Epub 2019 Apr 10. Oncogene. 2019. PMID: 30971761 Free PMC article.
-
Claudin-1 overexpression in intestinal epithelial cells enhances susceptibility to adenamatous polyposis coli-mediated colon tumorigenesis.Mol Cancer. 2014 Jul 6;13:167. doi: 10.1186/1476-4598-13-167. Mol Cancer. 2014. PMID: 24997475 Free PMC article.
-
Early-stage blocking of Notch signaling inhibits the depletion of goblet cells in dextran sodium sulfate-induced colitis in mice.J Gastroenterol. 2010 Jun;45(6):608-17. doi: 10.1007/s00535-010-0210-z. Epub 2010 Feb 19. J Gastroenterol. 2010. PMID: 20169455
-
The role of the Notch signalling pathway in the pathogenesis of ulcerative colitis: from the perspective of intestinal mucosal barrier.Front Med (Lausanne). 2024 Jan 5;10:1333531. doi: 10.3389/fmed.2023.1333531. eCollection 2023. Front Med (Lausanne). 2024. PMID: 38249980 Free PMC article. Review.
-
Colorectal keratins: Integrating nutrition, metabolism and colorectal health.Semin Cell Dev Biol. 2022 Aug;128:103-111. doi: 10.1016/j.semcdb.2021.08.010. Epub 2021 Sep 1. Semin Cell Dev Biol. 2022. PMID: 34481710 Review.
Cited by
-
Anti-inflammatory and glial response maintain normal colon function in trimethyltin-treated rats.Histochem Cell Biol. 2024 Dec;162(6):477-486. doi: 10.1007/s00418-024-02320-x. Epub 2024 Aug 22. Histochem Cell Biol. 2024. PMID: 39172242
-
The Role of Gut Barrier Dysfunction and Microbiome Dysbiosis in Colorectal Cancer Development.Front Oncol. 2021 Apr 15;11:626349. doi: 10.3389/fonc.2021.626349. eCollection 2021. Front Oncol. 2021. PMID: 33937029 Free PMC article. Review.
-
Cocaine Induces Inflammatory Gut Milieu by Compromising the Mucosal Barrier Integrity and Altering the Gut Microbiota Colonization.Sci Rep. 2019 Aug 21;9(1):12187. doi: 10.1038/s41598-019-48428-2. Sci Rep. 2019. PMID: 31434922 Free PMC article.
-
QingBai decoction regulates intestinal permeability of dextran sulphate sodium-induced colitis through the modulation of notch and NF-κB signalling.Cell Prolif. 2019 Mar;52(2):e12547. doi: 10.1111/cpr.12547. Epub 2019 Jan 18. Cell Prolif. 2019. PMID: 30657238 Free PMC article.
-
Upregulated claudin-1 expression promotes colitis-associated cancer by promoting β-catenin phosphorylation and activation in Notch/p-AKT-dependent manner.Oncogene. 2019 Jun;38(26):5321-5337. doi: 10.1038/s41388-019-0795-5. Epub 2019 Apr 10. Oncogene. 2019. PMID: 30971761 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- DK088902/DK/NIDDK NIH HHS/United States
- P30CA68485/CA/NCI NIH HHS/United States
- P50 CA095103/CA/NCI NIH HHS/United States
- R01069457/PHS HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- CA124977/CA/NCI NIH HHS/United States
- R25 GM062459/GM/NIGMS NIH HHS/United States
- R01 CA158472/CA/NCI NIH HHS/United States
- R01 DK088902/DK/NIDDK NIH HHS/United States
- R01 CA124977/CA/NCI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 CA069457/CA/NCI NIH HHS/United States
- R01CA158472/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
