miR-29 targets Akt3 to reduce proliferation and facilitate differentiation of myoblasts in skeletal muscle development
- PMID: 23764849
- PMCID: PMC3698551
- DOI: 10.1038/cddis.2013.184
miR-29 targets Akt3 to reduce proliferation and facilitate differentiation of myoblasts in skeletal muscle development
Abstract
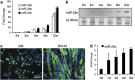
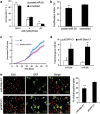
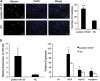
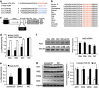
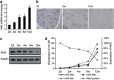
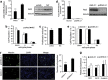
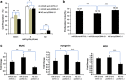
MicroRNAs (miRNAs) are a type of endogenous noncoding small RNAs involved in the regulation of multiple biological processes. Recently, miR-29 was found to participate in myogenesis. However, the underlying mechanisms by which miR-29 promotes myogenesis have not been identified. We found here that miR-29 was significantly upregulated with age in postnatal mouse skeletal muscle and during muscle differentiation. Overexpression of miR-29 inhibited mouse C2C12 myoblast proliferation and promoted myotube formation. miR-29 specifically targeted Akt3, a member of the serine/threonine protein kinase family responsive to growth factor cell signaling, to result in its post-transcriptional downregulation. Furthermore, knockdown of Akt3 by siRNA significantly inhibited the proliferation of C2C12 cells, and conversely, overexpression of Akt3 suppressed their differentiation. Collectively and given the inverse endogenous expression pattern of rising miR-29 levels and decreasing Akt3 protein levels with age in mouse skeletal muscle, we propose a novel mechanism in which miR-29 modulates growth and promotes differentiation of skeletal muscle through the post-transcriptional downregulation of Akt3.
Figures
Similar articles
-
Insulin-like growth factor-1 receptor is regulated by microRNA-133 during skeletal myogenesis.PLoS One. 2011;6(12):e29173. doi: 10.1371/journal.pone.0029173. Epub 2011 Dec 15. PLoS One. 2011. PMID: 22195016 Free PMC article.
-
Regulation of IRS1/Akt insulin signaling by microRNA-128a during myogenesis.J Cell Sci. 2013 Jun 15;126(Pt 12):2678-91. doi: 10.1242/jcs.119966. Epub 2013 Apr 19. J Cell Sci. 2013. PMID: 23606743 Free PMC article.
-
Guanidinoacetic Acid Regulates Myogenic Differentiation and Muscle Growth Through miR-133a-3p and miR-1a-3p Co-mediated Akt/mTOR/S6K Signaling Pathway.Int J Mol Sci. 2018 Sep 19;19(9):2837. doi: 10.3390/ijms19092837. Int J Mol Sci. 2018. PMID: 30235878 Free PMC article.
-
miR-29a suppresses growth and metastasis in papillary thyroid carcinoma by targeting AKT3.Tumour Biol. 2016 Mar;37(3):3987-96. doi: 10.1007/s13277-015-4165-9. Epub 2015 Oct 19. Tumour Biol. 2016. PMID: 26482618
-
Effects of microRNAs on skeletal muscle development.Gene. 2018 Aug 20;668:107-113. doi: 10.1016/j.gene.2018.05.039. Epub 2018 May 25. Gene. 2018. PMID: 29775754 Review.
Cited by
-
Targeting miR-29 mitigates skeletal senescence and bolsters therapeutic potential of mesenchymal stromal cells.Cell Rep Med. 2024 Aug 20;5(8):101665. doi: 10.1016/j.xcrm.2024.101665. Cell Rep Med. 2024. PMID: 39168101 Free PMC article.
-
The role of microRNAs in skeletal muscle health and disease.Front Biosci (Landmark Ed). 2015 Jan 1;20(1):37-77. doi: 10.2741/4298. Front Biosci (Landmark Ed). 2015. PMID: 25553440 Free PMC article. Review.
-
MicroRNA-29a attenuates CD8 T cell exhaustion and induces memory-like CD8 T cells during chronic infection.Proc Natl Acad Sci U S A. 2022 Apr 26;119(17):e2106083119. doi: 10.1073/pnas.2106083119. Epub 2022 Apr 21. Proc Natl Acad Sci U S A. 2022. PMID: 35446623 Free PMC article.
-
A Systematic Review of miR-29 in Cancer.Mol Ther Oncolytics. 2018 Dec 31;12:173-194. doi: 10.1016/j.omto.2018.12.011. eCollection 2019 Mar 29. Mol Ther Oncolytics. 2018. PMID: 30788428 Free PMC article. Review.
-
MicroRNA-29 induces cellular senescence in aging muscle through multiple signaling pathways.Aging (Albany NY). 2014 Mar;6(3):160-75. doi: 10.18632/aging.100643. Aging (Albany NY). 2014. PMID: 24659628 Free PMC article.
References
-
- Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. - PubMed
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–450. - PubMed
-
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

