Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors
- PMID: 23759113
- PMCID: PMC3706762
- DOI: 10.1186/scrt218
Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors
Abstract
Introduction: Platelet-rich plasma (PRP) is nowadays widely applied in different clinical scenarios, such as orthopedics, ophthalmology and healing therapies, as a growth factor pool for improving tissue regeneration. Studies into its clinical efficiency are not conclusive and one of the main reasons for this is that different PRP preparations are used, eliciting different responses that cannot be compared. Platelet quantification and the growth factor content definition must be defined in order to understand molecular mechanisms behind PRP regenerative strength. Standardization of PRP preparations is thus urgently needed.
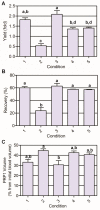
Methods: PRP was prepared by centrifugation varying the relative centrifugal force, temperature, and time. Having quantified platelet recovery and yield, the two-step procedure that rendered the highest output was chosen and further analyzed. Cytokine content was determined in different fractions obtained throughout the whole centrifugation procedure.
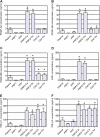
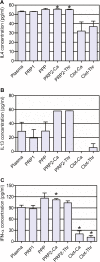
Results: Our method showed reproducibility when applied to different blood donors. We recovered 46.9 to 69.5% of total initial platelets and the procedure resulted in a 5.4-fold to 7.3-fold increase in platelet concentration (1.4 × 10(6) to 1.9 × 10(6) platelets/μl). Platelets were highly purified, because only <0.3% from the initial red blood cells and leukocytes was present in the final PRP preparation. We also quantified growth factors, cytokines and chemokines secreted by the concentrated platelets after activation with calcium and calcium/thrombin. High concentrations of platelet-derived growth factor, endothelial growth factor and transforming growth factor (TGF) were secreted, together with the anti-inflammatory and proinflammatory cytokines interleukin (IL)-4, IL-8, IL-13, IL-17, tumor necrosis factor (TNF)-α and interferon (IFN)-α. No cytokines were secreted before platelet activation. TGF-β3 and IFNγ were not detected in any studied fraction. Clots obtained after platelet coagulation retained a high concentration of several growth factors, including platelet-derived growth factor and TGF.
Conclusions: Our study resulted in a consistent PRP preparation method that yielded a cytokine and growth factor pool from different donors with high reproducibility. These findings support the use of PRP in therapies aiming for tissue regeneration, and its content characterization will allow us to understand and improve the clinical outcomes.
Figures

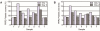
Similar articles
-
Characterization of Growth Factors, Cytokines, and Chemokines in Bone Marrow Concentrate and Platelet-Rich Plasma: A Prospective Analysis.Am J Sports Med. 2019 Jul;47(9):2174-2187. doi: 10.1177/0363546519832003. Epub 2019 Apr 29. Am J Sports Med. 2019. PMID: 31034242
-
Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma.Am J Sports Med. 2011 Oct;39(10):2135-40. doi: 10.1177/0363546511417792. Epub 2011 Aug 16. Am J Sports Med. 2011. PMID: 21846925
-
Short term storage stability at room temperature of two different platelet-rich plasma preparations from equine donors and potential impact on growth factor concentrations.BMC Vet Res. 2017 Jan 5;13(1):7. doi: 10.1186/s12917-016-0920-4. BMC Vet Res. 2017. PMID: 28056978 Free PMC article.
-
Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing.Int J Mol Sci. 2024 Jul 19;25(14):7914. doi: 10.3390/ijms25147914. Int J Mol Sci. 2024. PMID: 39063156 Free PMC article. Review.
-
Advances in separation methods for the use of platelet-rich fibrin in tissue repair: an integrative review.Gen Dent. 2023 Mar-Apr;71(2):65-69. Gen Dent. 2023. PMID: 36825976 Review.
Cited by
-
Efficacy of a Semi Automated Commercial Closed System for Autologous Leukocyte- and Platelet-Rich Plasma (l-prp) Production in Dogs: A Preliminary Study.Animals (Basel). 2020 Aug 4;10(8):1342. doi: 10.3390/ani10081342. Animals (Basel). 2020. PMID: 32759643 Free PMC article.
-
New strategy of personalized tissue regeneration: when autologous platelet concentrates encounter biomaterials.Front Bioeng Biotechnol. 2023 Nov 22;11:1297357. doi: 10.3389/fbioe.2023.1297357. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38076421 Free PMC article. Review.
-
Safety and Efficacy of Ultrasound-Guided Perineural Hydrodissection as a Minimally Invasive Treatment in Carpal Tunnel Syndrome: A Systematic Review.J Pers Med. 2024 Jan 30;14(2):154. doi: 10.3390/jpm14020154. J Pers Med. 2024. PMID: 38392587 Free PMC article. Review.
-
[Optimized preparation method of leukocytes-rich platelet-rich plasma by varying conditions during centrifugation].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020 Aug 15;34(8):1025-1030. doi: 10.7507/1002-1892.201911054. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020. PMID: 32794673 Free PMC article. Chinese.
-
Platelet gel: a new therapeutic tool with great potential.Blood Transfus. 2017 Jul;15(4):333-340. doi: 10.2450/2016.0038-16. Epub 2016 Jul 25. Blood Transfus. 2017. PMID: 27483482 Free PMC article. Review.
References
-
- Mazzocca AD, McCarthy MB, Chowaniec DM, Dugdale EM, Hansen D, Cote MP, Bradley JP, Romeo AA, Arciero RA, Beitzel K. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40:1742–1749. doi: 10.1177/0363546512452713. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

