Filter gate closure inhibits ion but not water transport through potassium channels
- PMID: 23754382
- PMCID: PMC3696751
- DOI: 10.1073/pnas.1304714110
Filter gate closure inhibits ion but not water transport through potassium channels
Abstract
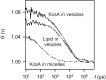
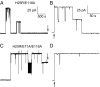
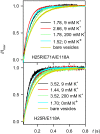
The selectivity filter of K(+) channels is conserved throughout all kingdoms of life. Carbonyl groups of highly conserved amino acids point toward the lumen to act as surrogates for the water molecules of K(+) hydration. Ion conductivity is abrogated if some of these carbonyl groups flip out of the lumen, which happens (i) in the process of C-type inactivation or (ii) during filter collapse in the absence of K(+). Here, we show that K(+) channels remain permeable to water, even after entering such an electrically silent conformation. We reconstituted fluorescently labeled and constitutively open mutants of the bacterial K(+) channel KcsA into lipid vesicles that were either C-type inactivating or noninactivating. Fluorescence correlation spectroscopy allowed us to count both the number of proteoliposomes and the number of protein-containing micelles after solubilization, providing the number of reconstituted channels per proteoliposome. Quantification of the per-channel increment in proteoliposome water permeability with the aid of stopped-flow experiments yielded a unitary water permeability pf of (6.9 ± 0.6) × 10(-13) cm(3)⋅s(-1) for both mutants. "Collapse" of the selectivity filter upon K(+) removal did not alter pf and was fully reversible, as demonstrated by current measurements through planar bilayers in a K(+)-containing medium to which K(+)-free proteoliposomes were fused. Water flow through KcsA is halved by 200 mM K(+) in the aqueous solution, which indicates an effective K(+) dissociation constant in that range for a singly occupied channel. This questions the widely accepted hypothesis that multiple K(+) ions in the selectivity filter act to mutually destabilize binding.
Keywords: aquaporin; brain water homeostasis; knock-on mechanism; membrane channels; protein reconstitution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Molecular determinants of gating at the potassium-channel selectivity filter.Nat Struct Mol Biol. 2006 Apr;13(4):311-8. doi: 10.1038/nsmb1069. Epub 2006 Mar 12. Nat Struct Mol Biol. 2006. PMID: 16532009
-
Pore hydration states of KcsA potassium channels in membranes.J Biol Chem. 2015 Oct 30;290(44):26765-75. doi: 10.1074/jbc.M115.661819. Epub 2015 Sep 14. J Biol Chem. 2015. PMID: 26370089 Free PMC article.
-
Voltage-dependent gating at the KcsA selectivity filter.Nat Struct Mol Biol. 2006 Apr;13(4):319-22. doi: 10.1038/nsmb1070. Epub 2006 Mar 12. Nat Struct Mol Biol. 2006. PMID: 16532008
-
Conformational dynamics in the selectivity filter of KcsA in response to potassium ion concentration.J Mol Biol. 2010 Aug 13;401(2):155-66. doi: 10.1016/j.jmb.2010.06.031. Epub 2010 Jun 19. J Mol Biol. 2010. PMID: 20600123 Free PMC article.
-
Unappreciated Roles for K+ Channels in Bacterial Physiology.Trends Microbiol. 2021 Oct;29(10):942-950. doi: 10.1016/j.tim.2020.11.005. Epub 2020 Dec 5. Trends Microbiol. 2021. PMID: 33288383 Free PMC article. Review.
Cited by
-
Water in Nanopores and Biological Channels: A Molecular Simulation Perspective.Chem Rev. 2020 Sep 23;120(18):10298-10335. doi: 10.1021/acs.chemrev.9b00830. Epub 2020 Aug 25. Chem Rev. 2020. PMID: 32841020 Free PMC article. Review.
-
Anomalous X-ray diffraction studies of ion transport in K+ channels.Nat Commun. 2018 Oct 31;9(1):4540. doi: 10.1038/s41467-018-06957-w. Nat Commun. 2018. PMID: 30382100 Free PMC article.
-
Ion conductivity of the bacterial translocation channel SecYEG engaged in translocation.J Biol Chem. 2014 Aug 29;289(35):24611-6. doi: 10.1074/jbc.M114.588491. Epub 2014 Jul 11. J Biol Chem. 2014. PMID: 25016015 Free PMC article.
-
Targeting secondary injury in intracerebral haemorrhage--perihaematomal oedema.Nat Rev Neurol. 2015 Feb;11(2):111-22. doi: 10.1038/nrneurol.2014.264. Epub 2015 Jan 27. Nat Rev Neurol. 2015. PMID: 25623787 Review.
-
Exploring the Dynamics of the TWIK-1 Channel.Biophys J. 2016 Aug 23;111(4):775-784. doi: 10.1016/j.bpj.2016.07.009. Biophys J. 2016. PMID: 27558721 Free PMC article.
References
-
- Bezanilla F. The action potential: From voltage-gated conductances to molecular structures. Biol Res. 2006;39(3):425–435. - PubMed
-
- Miller C. See potassium run. Nature. 2001;414(6859):23–24. - PubMed
-
- Doyle DA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280(5360):69–77. - PubMed
-
- Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414(6859):43–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

