Hyodeoxycholic acid improves HDL function and inhibits atherosclerotic lesion formation in LDLR-knockout mice
- PMID: 23752203
- PMCID: PMC3752541
- DOI: 10.1096/fj.12-223008
Hyodeoxycholic acid improves HDL function and inhibits atherosclerotic lesion formation in LDLR-knockout mice
Abstract
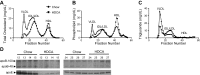
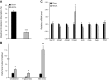
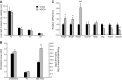
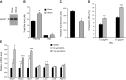
We examined the effects of a natural secondary bile acid, hyodeoxycholic acid (HDCA), on lipid metabolism and atherosclerosis in LDL receptor-null (LDLRKO) mice. Female LDLRKO mice were maintained on a Western diet for 8 wk and then divided into 2 groups that received chow, or chow + 1.25% HDCA, diets for 15 wk. We observed that mice fed the HDCA diet were leaner and exhibited a 37% (P<0.05) decrease in fasting plasma glucose level. HDCA supplementation significantly decreased atherosclerotic lesion size at the aortic root region, the entire aorta, and the innominate artery by 44% (P<0.0001), 48% (P<0.01), and 94% (P<0.01), respectively, as compared with the chow group. Plasma VLDL/IDL/LDL cholesterol levels were significantly decreased, by 61% (P<0.05), in the HDCA group as compared with the chow diet group. HDCA supplementation decreased intestinal cholesterol absorption by 76% (P<0.0001) as compared with the chow group. Furthermore, HDL isolated from the HDCA group exhibited significantly increased ability to mediate cholesterol efflux ex vivo as compared with HDL of the chow diet group. In addition, HDCA significantly increased the expression of genes involved in cholesterol efflux, such as Abca1, Abcg1, and Apoe, in a macrophage cell line. Thus, HDCA is a candidate for antiatherosclerotic drug therapy.
Keywords: bile acid; high-density lipoprotein; intestinal cholesterol absorption; low-density lipoprotein; reverse cholesterol transport.
Figures

Similar articles
-
Hyodeoxycholic acid efficiently suppresses atherosclerosis formation and plasma cholesterol levels in mice.J Lipid Res. 2001 Aug;42(8):1250-6. J Lipid Res. 2001. PMID: 11483626
-
Liver ABCA1 deletion in LDLrKO mice does not impair macrophage reverse cholesterol transport or exacerbate atherogenesis.Arterioscler Thromb Vasc Biol. 2013 Oct;33(10):2288-96. doi: 10.1161/ATVBAHA.112.301110. Epub 2013 Jun 27. Arterioscler Thromb Vasc Biol. 2013. PMID: 23814116 Free PMC article.
-
Macrophage ABCG1 deletion disrupts lipid homeostasis in alveolar macrophages and moderately influences atherosclerotic lesion development in LDL receptor-deficient mice.Arterioscler Thromb Vasc Biol. 2006 Oct;26(10):2295-300. doi: 10.1161/01.ATV.0000237629.29842.4c. Epub 2006 Jul 20. Arterioscler Thromb Vasc Biol. 2006. PMID: 16857950
-
Suppression of diet-induced atherosclerosis in low density lipoprotein receptor knockout mice overexpressing lipoprotein lipase.Proc Natl Acad Sci U S A. 1996 Jul 9;93(14):7242-6. doi: 10.1073/pnas.93.14.7242. Proc Natl Acad Sci U S A. 1996. PMID: 8692976 Free PMC article.
-
microRNAs in lipoprotein metabolism and cardiometabolic disorders.Atherosclerosis. 2016 Mar;246:352-60. doi: 10.1016/j.atherosclerosis.2016.01.025. Epub 2016 Jan 18. Atherosclerosis. 2016. PMID: 26828754 Free PMC article. Review.
Cited by
-
Bioactive TNIIIA2 Sequence in Tenascin-C Is Responsible for Macrophage Foam Cell Transformation; Potential of FNIII14 Peptide Derived from Fibronectin in Suppression of Atherosclerotic Plaque Formation.Int J Mol Sci. 2024 Feb 2;25(3):1825. doi: 10.3390/ijms25031825. Int J Mol Sci. 2024. PMID: 38339104 Free PMC article.
-
Intermittent Hypoxia and Hypercapnia Alter Diurnal Rhythms of Luminal Gut Microbiome and Metabolome.mSystems. 2021 Jun 29;6(3):e0011621. doi: 10.1128/mSystems.00116-21. Online ahead of print. mSystems. 2021. PMID: 34184915 Free PMC article.
-
Enhancing milk quality and modulating rectal microbiota of dairy goats in starch-rich diet: the role of bile acid supplementation.J Anim Sci Biotechnol. 2024 Jan 22;15(1):7. doi: 10.1186/s40104-023-00957-7. J Anim Sci Biotechnol. 2024. PMID: 38247003 Free PMC article.
-
Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis.J Lipid Res. 2015 Jan;56(1):22-37. doi: 10.1194/jlr.M051680. Epub 2014 Nov 6. J Lipid Res. 2015. PMID: 25378658 Free PMC article.
-
Growth performance, bile acid profile, fecal microbiome and serum metabolomics of growing-finishing pigs fed diets with bile acids supplementation.J Anim Sci. 2023 Jan 3;101:skad393. doi: 10.1093/jas/skad393. J Anim Sci. 2023. PMID: 38006392 Free PMC article.
References
-
- Grundy S. M. (1988) HMG-CoA reductase inhibitors for treatment of hypercholesterolemia. N. Engl. J. Med. 319, 24–33 - PubMed
-
- Scandinavian Simvastatin Survival Study Group (1994) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344, 1383–1389 - PubMed
-
- Shepherd J., Cobbe S. M., Ford I., Isles C. G., Lorimer A. R., MacFarlane P. W., McKillop J. H., Packard C. J. (1995) Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 333, 1301–1307 - PubMed
-
- The LIPID Study Group (1998) Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N. Engl. J. Med. 339, 1349–1357 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

