The incredible journey: From megakaryocyte development to platelet formation
- PMID: 23751492
- PMCID: PMC3678154
- DOI: 10.1083/jcb.201304054
The incredible journey: From megakaryocyte development to platelet formation
Abstract
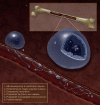
Circulating blood platelets are specialized cells that prevent bleeding and minimize blood vessel injury. Large progenitor cells in the bone marrow called megakaryocytes (MKs) are the source of platelets. MKs release platelets through a series of fascinating cell biological events. During maturation, they become polyploid and accumulate massive amounts of protein and membrane. Then, in a cytoskeletal-driven process, they extend long branching processes, designated proplatelets, into sinusoidal blood vessels where they undergo fission to release platelets. Given the need for platelets in many pathological situations, understanding how this process occurs is an active area of research with important clinical applications.
Figures
Similar articles
-
Interpreting the developmental dance of the megakaryocyte: a review of the cellular and molecular processes mediating platelet formation.Br J Haematol. 2014 Apr;165(2):227-36. doi: 10.1111/bjh.12758. Epub 2014 Feb 6. Br J Haematol. 2014. PMID: 24499183 Review.
-
Unraveling mechanisms that control platelet production.Semin Thromb Hemost. 2013 Feb;39(1):15-24. doi: 10.1055/s-0032-1331157. Epub 2012 Dec 24. Semin Thromb Hemost. 2013. PMID: 23266965 Review.
-
Ultrastructure of platelet formation by human megakaryocytes cultured with the Mpl ligand.Blood. 1997 Apr 1;89(7):2336-46. Blood. 1997. PMID: 9116277
-
Platelet production of megakaryocyte: A review with original observations on human in vivo cells and bone marrow.Ultrastruct Pathol. 2016 Jul-Aug;40(4):163-70. doi: 10.3109/01913123.2016.1170744. Epub 2016 May 9. Ultrastruct Pathol. 2016. PMID: 27159022 Review.
-
The birth of the platelet.J Thromb Haemost. 2003 Jul;1(7):1580-6. doi: 10.1046/j.1538-7836.2003.00331.x. J Thromb Haemost. 2003. PMID: 12871294 Review.
Cited by
-
Shear-mediated platelet activation in the free flow II: Evolving mechanobiological mechanisms reveal an identifiable signature of activation and a bi-directional platelet dyscrasia with thrombotic and bleeding features.J Biomech. 2021 Jun 23;123:110415. doi: 10.1016/j.jbiomech.2021.110415. Epub 2021 Apr 27. J Biomech. 2021. PMID: 34052772 Free PMC article. Review.
-
Comparative analysis of human ex vivo-generated platelets vs megakaryocyte-generated platelets in mice: a cautionary tale.Blood. 2015 Jun 4;125(23):3627-36. doi: 10.1182/blood-2014-08-593053. Epub 2015 Apr 7. Blood. 2015. PMID: 25852052 Free PMC article.
-
Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming.Nat Commun. 2016 Apr 7;7:11208. doi: 10.1038/ncomms11208. Nat Commun. 2016. PMID: 27052461 Free PMC article.
-
Human Red Blood Cells Modulate Cytokine Expression in Monocytes/Macrophages Under Anoxic Conditions.Front Physiol. 2021 Feb 18;12:632682. doi: 10.3389/fphys.2021.632682. eCollection 2021. Front Physiol. 2021. PMID: 33679443 Free PMC article.
-
Insights into primary immune deficiency from quantitative microscopy.J Allergy Clin Immunol. 2015 Nov;136(5):1150-62. doi: 10.1016/j.jaci.2015.03.049. Epub 2015 Jun 13. J Allergy Clin Immunol. 2015. PMID: 26078103 Free PMC article. Review.
References
-
- Aster R.H. 1967. Studies of the mechanism of “hypersplenic” thrombocytopenia in rats. J. Lab. Clin. Med. 70:736–751 - PubMed
-
- Avecilla S.T., Hattori K., Heissig B., Tejada R., Liao F., Shido K., Jin D.K., Dias S., Zhang F., Hartman T.E., et al. 2004. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 10:64–71 10.1038/nm973 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

