Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling
- PMID: 23749232
- PMCID: PMC3859297
- DOI: 10.1038/nm.3194
Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling
Abstract
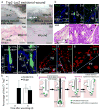
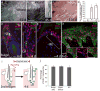
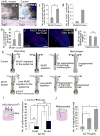
During wound healing, stem cells provide functional mature cells to meet acute demands for tissue regeneration. Simultaneously, the tissue must maintain a pool of stem cells to sustain its future regeneration capability. However, how these requirements are balanced in response to injury is unknown. Here we demonstrate that after wounding or ultraviolet type B irradiation, melanocyte stem cells (McSCs) in the hair follicle exit the stem cell niche before their initial cell division, potentially depleting the pool of these cells. We also found that McSCs migrate to the epidermis in a melanocortin 1 receptor (Mc1r)-dependent manner and differentiate into functional epidermal melanocytes, providing a pigmented protective barrier against ultraviolet irradiation over the damaged skin. These findings provide an example in which stem cell differentiation due to injury takes precedence over stem cell maintenance and show the potential for developing therapies for skin pigmentation disorders by manipulating McSCs.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Comment in
-
Migrating melanocyte stem cells: masters of disaster?Nat Med. 2013 Jul;19(7):818-9. doi: 10.1038/nm.3264. Nat Med. 2013. PMID: 23836222 No abstract available.
Similar articles
-
SPRY1 Deficiency in Keratinocytes Induces Follicular Melanocyte Stem Cell Migration to the Epidermis through p53/Stem Cell Factor/C-KIT Signaling.J Invest Dermatol. 2024 Oct;144(10):2255-2266.e4. doi: 10.1016/j.jid.2024.02.018. Epub 2024 Mar 8. J Invest Dermatol. 2024. PMID: 38462125
-
Distinct pigmentary and melanocortin 1 receptor-dependent components of cutaneous defense against ultraviolet radiation.PLoS Genet. 2007 Jan 12;3(1):e9. doi: 10.1371/journal.pgen.0030009. Epub 2006 Dec 1. PLoS Genet. 2007. PMID: 17222061 Free PMC article.
-
Hair follicle melanocyte precursors are awoken by ultraviolet radiation via a cell extrinsic mechanism.Photochem Photobiol Sci. 2015 Jun;14(6):1179-89. doi: 10.1039/c5pp00098j. Photochem Photobiol Sci. 2015. PMID: 25966309
-
Skin pigmentation and its control: From ultraviolet radiation to stem cells.Exp Dermatol. 2021 Apr;30(4):560-571. doi: 10.1111/exd.14260. Epub 2020 Dec 24. Exp Dermatol. 2021. PMID: 33320376 Free PMC article. Review.
-
Melanocortins and the melanocortin 1 receptor, moving translationally towards melanoma prevention.Arch Biochem Biophys. 2014 Dec 1;563:4-12. doi: 10.1016/j.abb.2014.07.002. Epub 2014 Jul 11. Arch Biochem Biophys. 2014. PMID: 25017567 Review.
Cited by
-
Sexual dimorphism in melanocyte stem cell behavior reveals combinational therapeutic strategies for cutaneous repigmentation.Nat Commun. 2024 Jan 27;15(1):796. doi: 10.1038/s41467-024-45034-3. Nat Commun. 2024. PMID: 38280858 Free PMC article.
-
Stem cells and targeted approaches to melanoma cure.Mol Aspects Med. 2014 Oct;39:33-49. doi: 10.1016/j.mam.2013.10.003. Epub 2013 Oct 19. Mol Aspects Med. 2014. PMID: 24145241 Free PMC article. Review.
-
β-Catenin-regulated myeloid cell adhesion and migration determine wound healing.J Clin Invest. 2014 Jun;124(6):2599-610. doi: 10.1172/JCI62059. Epub 2014 May 16. J Clin Invest. 2014. PMID: 24837430 Free PMC article.
-
Melanocyte stem cells in the skin: Origin, biological characteristics, homeostatic maintenance and therapeutic potential.Clin Transl Med. 2024 May;14(5):e1720. doi: 10.1002/ctm2.1720. Clin Transl Med. 2024. PMID: 38778457 Free PMC article. Review.
-
Recent advances in molecular mechanisms of skin wound healing and its treatments.Front Immunol. 2024 May 21;15:1395479. doi: 10.3389/fimmu.2024.1395479. eCollection 2024. Front Immunol. 2024. PMID: 38835782 Free PMC article. Review.
References
-
- Nishimura EK, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. - PubMed
-
- Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–850. - PubMed
-
- Hirobe T. Histochemical survey of the distribution of the epidermal melanoblasts and melanocytes in the mouse during fetal and postnatal periods. Anat Rec. 1984;208:589–594. - PubMed
-
- Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011;24:401–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

