Structural Modifications of Bacterial Lipopolysaccharide that Facilitate Gram-Negative Bacteria Evasion of Host Innate Immunity
- PMID: 23745121
- PMCID: PMC3662973
- DOI: 10.3389/fimmu.2013.00109
Structural Modifications of Bacterial Lipopolysaccharide that Facilitate Gram-Negative Bacteria Evasion of Host Innate Immunity
Abstract
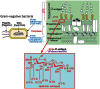
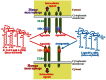
Bacterial lipopolysaccharide (LPS), a cell wall component characteristic of Gram-negative bacteria, is a representative pathogen-associated molecular pattern that allows mammalian cells to recognize bacterial invasion and trigger innate immune responses. The polysaccharide moiety of LPS primary plays protective roles for bacteria such as prevention from complement attacks or camouflage with common host carbohydrate residues. The lipid moiety, termed lipid A, is recognized by the Toll-like receptor 4 (TLR4)/MD-2 complex, which transduces signals for activation of host innate immunity. The basic structure of lipid A is a glucosamine disaccharide substituted by phosphate groups and acyl groups. Lipid A with six acyl groups (hexa-acylated form) has been indicated to be a strong stimulator of the TLR4/MD-2 complex. This type of lipid A is conserved among a wide variety of Gram-negative bacteria, and those bacteria are easily recognized by host cells for activation of defensive innate immune responses. Modifications of the lipid A structure to less-acylated forms have been observed in some bacterial species, and those forms are poor stimulators of the TLR4/MD-2 complex. Such modifications are thought to facilitate bacterial evasion of host innate immunity, thereby enhancing pathogenicity. This hypothesis is supported by studies of Yersinia pestis LPS, which contains hexa-acylated lipid A when the bacterium grows at 27°C (the temperature of the vector flea), and shifts to contain less-acylated forms when grown at the human body temperature of 37°C. This alteration of lipid A forms following transmission of Y. pestis from fleas to humans contributes predominantly to the virulence of this bacterium over other virulence factors. A similar role for less-acylated lipid A forms has been indicated in some other bacterial species, such as Francisella tularensis, Helicobacter pylori, and Porphyromonas gingivalis, and further studies to explore this concept are expected.
Keywords: immune evasion; innate immunity; less-acylated lipid A; modification of lipopolysaccharide.
Figures

Similar articles
-
Evasion of human innate immunity without antagonizing TLR4 by mutant Salmonella enterica serovar Typhimurium having penta-acylated lipid A.Innate Immun. 2012 Oct;18(5):764-73. doi: 10.1177/1753425912440599. Epub 2012 Mar 14. Innate Immun. 2012. PMID: 22419537
-
Bordetella pertussis Lipid A Recognition by Toll-like Receptor 4 and MD-2 Is Dependent on Distinct Charged and Uncharged Interfaces.J Biol Chem. 2015 May 22;290(21):13440-53. doi: 10.1074/jbc.M115.653881. Epub 2015 Apr 2. J Biol Chem. 2015. PMID: 25837248 Free PMC article.
-
Activation of Human Toll-like Receptor 4 (TLR4)·Myeloid Differentiation Factor 2 (MD-2) by Hypoacylated Lipopolysaccharide from a Clinical Isolate of Burkholderia cenocepacia.J Biol Chem. 2015 Aug 28;290(35):21305-19. doi: 10.1074/jbc.M115.649087. Epub 2015 Jul 9. J Biol Chem. 2015. PMID: 26160169 Free PMC article.
-
Reviewing and identifying amino acids of human, murine, canine and equine TLR4 / MD-2 receptor complexes conferring endotoxic innate immunity activation by LPS/lipid A, or antagonistic effects by Eritoran, in contrast to species-dependent modulation by lipid IVa.Comput Struct Biotechnol J. 2013 Apr 5;5:e201302012. doi: 10.5936/csbj.201302012. eCollection 2013. Comput Struct Biotechnol J. 2013. PMID: 24688705 Free PMC article. Review.
-
Recognition of lipid A variants by the TLR4-MD-2 receptor complex.Front Cell Infect Microbiol. 2013 Feb 12;3:3. doi: 10.3389/fcimb.2013.00003. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 23408095 Free PMC article. Review.
Cited by
-
Shotgun Bacterial Lipid A Analysis Using Routine MALDI-TOF Mass Spectrometry.Methods Mol Biol. 2021;2306:275-283. doi: 10.1007/978-1-0716-1410-5_18. Methods Mol Biol. 2021. PMID: 33954953
-
Periplasmic Targets for the Development of Effective Antimicrobials against Gram-Negative Bacteria.ACS Infect Dis. 2020 Sep 11;6(9):2337-2354. doi: 10.1021/acsinfecdis.0c00384. Epub 2020 Aug 24. ACS Infect Dis. 2020. PMID: 32786281 Free PMC article. Review.
-
Differential Rickettsial Transcription in Bloodfeeding and Non-Bloodfeeding Arthropod Hosts.PLoS One. 2016 Sep 23;11(9):e0163769. doi: 10.1371/journal.pone.0163769. eCollection 2016. PLoS One. 2016. PMID: 27662479 Free PMC article.
-
Helminth infection is associated with dampened cytokine responses to viral and bacterial stimulations in Tsimane forager-horticulturalists.Evol Med Public Health. 2021 Oct 26;9(1):349-359. doi: 10.1093/emph/eoab035. eCollection 2021. Evol Med Public Health. 2021. PMID: 34868595 Free PMC article.
-
NRF2 activation by 2-methoxycinnamaldehyde attenuates inflammatory responses in macrophages via enhancing autophagy flux.BMB Rep. 2022 Aug;55(8):407-412. doi: 10.5483/BMBRep.2022.55.8.065. BMB Rep. 2022. PMID: 35725014 Free PMC article.
References
-
- Akashi S., Shimazu R., Ogata H., Nagai Y., Takeda K., Kimoto M., et al. (2000). Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 164, 3471–3475 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

