Human cytomegalovirus (HCMV) glycoprotein gB promotes virus entry in trans acting as the viral fusion protein rather than as a receptor-binding protein
- PMID: 23736286
- PMCID: PMC3685210
- DOI: 10.1128/mBio.00332-13
Human cytomegalovirus (HCMV) glycoprotein gB promotes virus entry in trans acting as the viral fusion protein rather than as a receptor-binding protein
Abstract
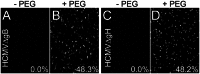
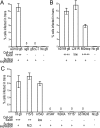
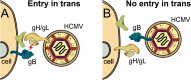
ABSTRACT Human cytomegalovirus (HCMV) glycoproteins gB and gH/gL are both necessary and sufficient for cell-cell fusion. However, it is not clear what roles these glycoproteins play in virus entry, whether acting directly in membrane fusion or in binding receptors. With other herpesviruses, it appears that gB is the fusion protein and is triggered by gH/gL, which, in some cases, binds receptors. However, for HCMV, there is published evidence that gB binds cellular ligands necessary to promote virus entry into or signaling of cells. Most mechanistic information on herpesvirus fusion proteins involves cell-cell fusion assays, which do not allow a determination of whether gB or gH/gL in the virion envelope must be oriented toward cellular membranes that contain receptors. Here, we showed that HCMV virions lacking gB were unable to enter normal cells but entered cells that expressed gB. Analyses of gB mutants lacking the cytoplasmic domain or with substitutions in putative "fusion loops" provided evidence that gB fusion activity was required for this "entry in trans." In gB-mediated entry in trans, gB is oriented toward the virion envelope that apparently lacks receptors, arguing against an essential role for gB in binding receptors or signaling molecules. In contrast, particles lacking gH/gL did not enter cells expressing gH/gL, apparently because gH/gL must be oriented toward cellular membranes (which have receptors). Coupled with our previous interference studies, in which gH/gL expressed in cells blocked HCMV entry, our findings here support the hypothesis that HCMV gH/gL binds cellular receptors before triggering gB, which acts as the fusion protein. IMPORTANCE Human cytomegalovirus (HCMV) produces major disease in neonates and immunosuppressed transplant patients. As with other herpesviruses, HCMV requires two membrane glycoproteins, gB and gH/gL, to enter host cells. However, it has not been clear how gB and gH/gL function in two steps of the HCMV entry pathway, i.e., (i) binding of cellular receptors and (ii) fusion of the virion envelope with cellular membranes. There are studies that suggest that HCMV gB is required for receptor binding and other studies suggesting that gH/gL is the receptor binding protein and gB is the fusion protein. Here, we show that HCMV virions lacking gB can enter cells that express gB in cellular membranes. In contrast, virus particles lacking gH/gL could not enter cells expressing gH/gL. Our study supports the hypothesis that gB is the fusion protein and gH/gL acts upstream of gB to bind receptors and then activate gB for fusion.
Figures


Similar articles
-
Human Cytomegalovirus gH/gL/gO Promotes the Fusion Step of Entry into All Cell Types, whereas gH/gL/UL128-131 Broadens Virus Tropism through a Distinct Mechanism.J Virol. 2015 Sep;89(17):8999-9009. doi: 10.1128/JVI.01325-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085146 Free PMC article.
-
Scanning Mutagenesis of Human Cytomegalovirus Glycoprotein gH/gL.J Virol. 2015 Dec 9;90(5):2294-305. doi: 10.1128/JVI.01875-15. J Virol. 2015. PMID: 26656708 Free PMC article.
-
Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-α as a key for entry.PLoS Pathog. 2017 Apr 12;13(4):e1006281. doi: 10.1371/journal.ppat.1006281. eCollection 2017 Apr. PLoS Pathog. 2017. PMID: 28403202 Free PMC article.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
-
Two Sides to Every Story: Herpes Simplex Type-1 Viral Glycoproteins gB, gD, gH/gL, gK, and Cellular Receptors Function as Key Players in Membrane Fusion.Viruses. 2021 Sep 16;13(9):1849. doi: 10.3390/v13091849. Viruses. 2021. PMID: 34578430 Free PMC article. Review.
Cited by
-
Human Cytomegalovirus Cell Tropism and Host Cell Receptors.Vaccines (Basel). 2019 Jul 22;7(3):70. doi: 10.3390/vaccines7030070. Vaccines (Basel). 2019. PMID: 31336680 Free PMC article. Review.
-
UL34 Deletion Restricts Human Cytomegalovirus Capsid Formation and Maturation.Int J Mol Sci. 2022 May 21;23(10):5773. doi: 10.3390/ijms23105773. Int J Mol Sci. 2022. PMID: 35628580 Free PMC article.
-
Polymorphic Forms of Human Cytomegalovirus Glycoprotein O Protect against Neutralization of Fibroblast Entry by Antibodies Targeting Epitopes Defined by Glycoproteins H and L.Viruses. 2022 Jul 9;14(7):1508. doi: 10.3390/v14071508. Viruses. 2022. PMID: 35891489 Free PMC article.
-
Functional Relevance of the Transmembrane Domain and Cytoplasmic Tail of the Pseudorabies Virus Glycoprotein H for Membrane Fusion.J Virol. 2018 May 29;92(12):e00376-18. doi: 10.1128/JVI.00376-18. Print 2018 Jun 15. J Virol. 2018. PMID: 29618646 Free PMC article.
-
Selection of Human Cytomegalovirus Mutants with Resistance against PDGFRα-Derived Entry Inhibitors.Viruses. 2021 Jun 8;13(6):1094. doi: 10.3390/v13061094. Viruses. 2021. PMID: 34201364 Free PMC article.
References
-
- Compton T, Nepomuceno RR, Nowlin DM. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191:387–395 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
