Collagen IV-modified scaffolds improve islet survival and function and reduce time to euglycemia
- PMID: 23713524
- PMCID: PMC3807710
- DOI: 10.1089/ten.TEA.2013.0033
Collagen IV-modified scaffolds improve islet survival and function and reduce time to euglycemia
Abstract
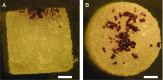
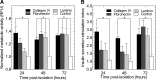
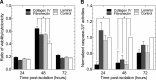
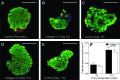
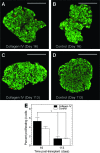
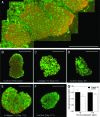
Islet transplantation on extracellular matrix (ECM) protein-modified biodegradable microporous poly(lactide-co-glycolide) scaffolds is a potential curative treatment for type 1 diabetes mellitus (T1DM). Collagen IV-modified scaffolds, relative to control scaffolds, significantly decreased the time required to restore euglycemia from 17 to 3 days. We investigated the processes by which collagen IV-modified scaffolds enhanced islet function and mediated early restoration of euglycemia post-transplantation. We characterized the effect of collagen IV-modified scaffolds on islet survival, metabolism, and insulin secretion in vitro and early- and intermediate-term islet mass and vascular density post-transplantation and correlated these with early restoration of euglycemia in a syngeneic mouse model. Control scaffolds maintained native islet morphologies and architectures as well as collagen IV-modified scaffolds in vivo. The islet size and vascular density increased, while β-cell proliferation decreased from day 16 to 113 post-transplantation. Collagen IV-modified scaffolds promoted islet cell viability and decreased early-stage apoptosis in islet cells in vitro-phenomena that coincided with enhanced islet metabolic function and glucose-stimulated insulin secretion. These findings suggest that collagen IV-modified scaffolds promote the early restoration of euglycemia post-transplantation by enhancing islet metabolism and glucose-stimulated insulin secretion. These studies of ECM proteins, in particular collagen IV, and islet function provide key insights for the engineering of a microenvironment that would serve as a platform for enhancing islet transplantation as a viable clinical therapy for T1DM.
Figures

Similar articles
-
Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation.Transplantation. 2008 May 27;85(10):1456-64. doi: 10.1097/TP.0b013e31816fc0ea. Transplantation. 2008. PMID: 18497687 Free PMC article.
-
Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel.Biomaterials. 2012 Oct;33(28):6691-7. doi: 10.1016/j.biomaterials.2012.06.015. Epub 2012 Jul 4. Biomaterials. 2012. PMID: 22766242 Free PMC article.
-
Macroporous biohybrid cryogels for co-housing pancreatic islets with mesenchymal stromal cells.Acta Biomater. 2016 Oct 15;44:178-87. doi: 10.1016/j.actbio.2016.08.007. Epub 2016 Aug 6. Acta Biomater. 2016. PMID: 27506126
-
Use of additives, scaffolds and extracellular matrix components for improvement of human pancreatic islet outcomes in vitro: A systematic review.Islets. 2017 Sep 3;9(5):73-86. doi: 10.1080/19382014.2017.1335842. Epub 2017 Jul 5. Islets. 2017. PMID: 28678625 Free PMC article. Review.
-
Collagen matrix support of pancreatic islet survival and function.Front Biosci (Landmark Ed). 2014 Jan 1;19(1):77-90. doi: 10.2741/4196. Front Biosci (Landmark Ed). 2014. PMID: 24389173 Review.
Cited by
-
The lncRNA BORG facilitates the survival and chemoresistance of triple-negative breast cancers.Oncogene. 2019 Mar;38(12):2020-2041. doi: 10.1038/s41388-018-0586-4. Epub 2018 Nov 22. Oncogene. 2019. PMID: 30467380 Free PMC article.
-
Enhancing islet transplantation using a biocompatible collagen-PDMS bioscaffold enriched with dexamethasone-microplates.Biofabrication. 2021 Apr 7;13(3):10.1088/1758-5090/abdcac. doi: 10.1088/1758-5090/abdcac. Biofabrication. 2021. PMID: 33455953 Free PMC article.
-
Therapeutic effects of Salvia miltiorrhiza injection combined with telmisartan in patients with diabetic nephropathy by influencing collagen IV and fibronectin: A case-control study.Exp Ther Med. 2018 Oct;16(4):3405-3412. doi: 10.3892/etm.2018.6654. Epub 2018 Aug 24. Exp Ther Med. 2018. PMID: 30233688 Free PMC article.
-
Enhancing human islet transplantation by localized release of trophic factors from PLG scaffolds.Am J Transplant. 2014 Jul;14(7):1523-32. doi: 10.1111/ajt.12742. Epub 2014 Jun 6. Am J Transplant. 2014. PMID: 24909237 Free PMC article.
-
In vivo capture and label-free detection of early metastatic cells.Nat Commun. 2015 Sep 8;6:8094. doi: 10.1038/ncomms9094. Nat Commun. 2015. PMID: 26348915 Free PMC article.
References
-
- Eiselein L. Schwartz H.J. Rutledge J.C. The challenge of type 1 diabetes mellitus. ILAR J. 2004;45:231. - PubMed
-
- Kay T.W. Thomas H.E. Harrison L.C. Allison J. The beta cell in autoimmune diabetes: many mechanisms and pathways of loss. Trends in endocrinology and metabolism. TEM. 2000;11:11. - PubMed
-
- Shapiro A.M. Lakey J.R. Ryan E.A. Korbutt G.S. Toth E. Warnock G.L. Kneteman N.M. Rajotte R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. - PubMed
-
- Ryan E.A. Paty B.W. Senior P.A. Bigam D. Alfadhli E. Kneteman N.M. Lakey J.R. Shapiro A.M. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060. - PubMed
-
- Hering B.J. Achieving and maintaining insulin independence in human islet transplant recipients. Transplantation. 2005;79:1296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

