Relaxin prevents cardiac fibroblast-myofibroblast transition via notch-1-mediated inhibition of TGF-β/Smad3 signaling
- PMID: 23704950
- PMCID: PMC3660557
- DOI: 10.1371/journal.pone.0063896
Relaxin prevents cardiac fibroblast-myofibroblast transition via notch-1-mediated inhibition of TGF-β/Smad3 signaling
Abstract
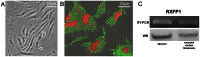
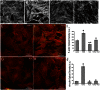
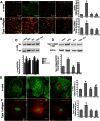
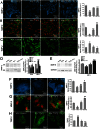
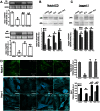
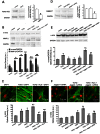
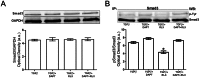
The hormone relaxin (RLX) is produced by the heart and has beneficial actions on the cardiovascular system. We previously demonstrated that RLX stimulates mouse neonatal cardiomyocyte growth, suggesting its involvement in endogenous mechanisms of myocardial histogenesis and regeneration. In the present study, we extended the experimentation by evaluating the effects of RLX on primary cultures of neonatal cardiac stromal cells. RLX inhibited TGF-β1-induced fibroblast-myofibroblast transition, as judged by its ability to down-regulate α-smooth muscle actin and type I collagen expression. We also found that the hormone up-regulated metalloprotease (MMP)-2 and MMP-9 expression and downregulated the tissue inhibitor of metalloproteinases (TIMP)-2 in TGF-β1-stimulated cells. Interestingly, the effects of RLX on cardiac fibroblasts involved the activation of Notch-1 pathway. Indeed, Notch-1 expression was significantly decreased in TGF-β1-stimulatedfibroblasts as compared to the unstimulated controls; this reduction was prevented by the addition of RLX to TGF-β1-stimulated cells. Moreover, pharmacological inhibition of endogenous Notch-1 signaling by N-3,5-difluorophenyl acetyl-L-alanyl-2-phenylglycine-1,1-dimethylethyl ester (DAPT), a γ-secretase specific inhibitor, as well as the silencing of Notch-1 ligand, Jagged-1, potentiated TGF-β1-induced myofibroblast differentiation and abrogated the inhibitory effects of RLX. Interestingly, RLX and Notch-1 exerted their inhibitory effects by interfering with TGF-β1 signaling, since the addition of RLX to TGF-β1-stimulated cells caused a significant decrease in Smad3 phosphorylation, a typical downstream event of TGF-β1 receptor activation, while the treatment with a prevented this effect. These data suggest that Notch signaling can down-regulate TGF-β1/Smad3-induced fibroblast-myofibroblast transition and that RLX could exert its well known anti-fibrotic action through the up-regulation of this pathway. In conclusion, the results of the present study beside supporting the role of RLX in the field of cardiac fibrosis, provide novel experimental evidence on the molecular mechanisms underlying its effects.
Conflict of interest statement
Figures
Similar articles
-
The anti-fibrotic actions of relaxin are mediated through AT2 R-associated protein phosphatases via RXFP1-AT2 R functional crosstalk in human cardiac myofibroblasts.FASEB J. 2020 Jun;34(6):8217-8233. doi: 10.1096/fj.201902506R. Epub 2020 Apr 16. FASEB J. 2020. PMID: 32297670
-
Inhibitory effects of relaxin on cardiac fibroblast-to-myofibroblast transition: an electrophysiological study.Exp Physiol. 2015 Jun;100(6):652-66. doi: 10.1113/EP085178. Epub 2015 May 13. Exp Physiol. 2015. PMID: 25786395
-
Serelaxin inhibits the profibrotic TGF-β1/IL-1β axis by targeting TLR-4 and the NLRP3 inflammasome in cardiac myofibroblasts.FASEB J. 2019 Dec;33(12):14717-14733. doi: 10.1096/fj.201901079RR. Epub 2019 Nov 5. FASEB J. 2019. PMID: 31689135
-
Investigations into the inhibitory effects of relaxin on renal myofibroblast differentiation.Ann N Y Acad Sci. 2009 Apr;1160:294-9. doi: 10.1111/j.1749-6632.2008.03823.x. Ann N Y Acad Sci. 2009. PMID: 19416207 Review.
-
Human Recombinant Relaxin (Serelaxin) as Anti-fibrotic Agent: Pharmacology, Limitations and Actual Perspectives.Curr Mol Med. 2022;22(3):196-208. doi: 10.2174/1566524021666210309113650. Curr Mol Med. 2022. PMID: 33687895 Review.
Cited by
-
International Union of Basic and Clinical Pharmacology. XCV. Recent advances in the understanding of the pharmacology and biological roles of relaxin family peptide receptors 1-4, the receptors for relaxin family peptides.Pharmacol Rev. 2015;67(2):389-440. doi: 10.1124/pr.114.009472. Pharmacol Rev. 2015. PMID: 25761609 Free PMC article. Review.
-
Serelaxin induces Notch1 signaling and alleviates hepatocellular damage in orthotopic liver transplantation.Am J Transplant. 2018 Jul;18(7):1755-1763. doi: 10.1111/ajt.14706. Epub 2018 Mar 23. Am J Transplant. 2018. PMID: 29464890 Free PMC article.
-
Therapeutic approaches for cardiac regeneration and repair.Nat Rev Cardiol. 2018 Oct;15(10):585-600. doi: 10.1038/s41569-018-0036-6. Nat Rev Cardiol. 2018. PMID: 29872165 Free PMC article. Review.
-
Research progress of knee fibrosis after anterior cruciate ligament reconstruction.Front Pharmacol. 2024 Oct 21;15:1493155. doi: 10.3389/fphar.2024.1493155. eCollection 2024. Front Pharmacol. 2024. PMID: 39498335 Free PMC article. Review.
-
Platelet-Rich Plasma Prevents In Vitro Transforming Growth Factor-β1-Induced Fibroblast to Myofibroblast Transition: Involvement of Vascular Endothelial Growth Factor (VEGF)-A/VEGF Receptor-1-Mediated Signaling †.Cells. 2018 Sep 19;7(9):142. doi: 10.3390/cells7090142. Cells. 2018. PMID: 30235859 Free PMC article.
References
-
- Samuel CS, Lekgabe ED, Mookerjee I (2007) The effects of relaxin on extracellular matrix remodeling in health and fibrotic disease. Adv Exp Med Biol 612: 88–103. - PubMed
-
- Hisaw FL (1926) Experimental relaxation of the pubic ligament of the guinea pig. Proc Soc Exp Biol Med 23: 661–663.
-
- Dschietzig T, Bartsch C, Baumann G, Stangl K (2006) Relaxin-a pleiotropic hormone and its emerging role for experimental and clinical therapeutics. Pharmacol Ther 112: 38–56. - PubMed
-
- Du XJ, Bathgate RA, Samuel CS, Dart AM, Summers RJ (2010) Cardiovascular effects of relaxin: from basic science to clinical therapy. Nat Rev Cardiol 7: 48–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

