Activation and regulation of the inflammasomes
- PMID: 23702978
- PMCID: PMC3807999
- DOI: 10.1038/nri3452
Activation and regulation of the inflammasomes
Abstract
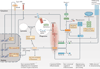
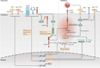
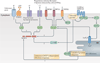
Inflammasomes are key signalling platforms that detect pathogenic microorganisms and sterile stressors, and that activate the highly pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18. In this Review, we discuss the complex regulatory mechanisms that facilitate a balanced but effective inflammasome-mediated immune response, and we highlight the similarities to another molecular signalling platform - the apoptosome - that monitors cellular health. Extracellular regulatory mechanisms are discussed, as well as the intracellular control of inflammasome assembly, for example, via ion fluxes, free radicals and autophagy.
Figures
Similar articles
-
IL-1β and IL-18: inflammatory markers or mediators of hypertension?Br J Pharmacol. 2014 Dec;171(24):5589-602. doi: 10.1111/bph.12876. Br J Pharmacol. 2014. PMID: 25117218 Free PMC article. Review.
-
The NLRP3 inflammasome in kidney disease and autoimmunity.Nephrology (Carlton). 2016 Sep;21(9):736-44. doi: 10.1111/nep.12785. Nephrology (Carlton). 2016. PMID: 27011059 Review.
-
The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1β.Exp Physiol. 2013 Feb;98(2):462-72. doi: 10.1113/expphysiol.2012.068338. Epub 2012 Jul 30. Exp Physiol. 2013. PMID: 22848083
-
Methylsulfonylmethane inhibits NLRP3 inflammasome activation.Cytokine. 2015 Feb;71(2):223-31. doi: 10.1016/j.cyto.2014.11.001. Epub 2014 Nov 21. Cytokine. 2015. PMID: 25461402
-
Cutting edge: the NLRP3 inflammasome links complement-mediated inflammation and IL-1β release.J Immunol. 2013 Aug 1;191(3):1006-10. doi: 10.4049/jimmunol.1300489. Epub 2013 Jul 1. J Immunol. 2013. PMID: 23817414 Free PMC article.
Cited by
-
Activators of SIRT1 in the kidney and protective effects of SIRT1 during acute kidney injury (AKI) (effect of SIRT1 activators on acute kidney injury).Clin Exp Nephrol. 2021 Aug;25(8):807-821. doi: 10.1007/s10157-021-02057-0. Epub 2021 Mar 29. Clin Exp Nephrol. 2021. PMID: 33779856 Review.
-
NLRP3 Inflammasome/Pyroptosis: A Key Driving Force in Diabetic Cardiomyopathy.Int J Mol Sci. 2022 Sep 13;23(18):10632. doi: 10.3390/ijms231810632. Int J Mol Sci. 2022. PMID: 36142531 Free PMC article. Review.
-
A proteomics perspective on viral DNA sensors in host defense and viral immune evasion mechanisms.J Mol Biol. 2015 Jun 5;427(11):1995-2012. doi: 10.1016/j.jmb.2015.02.016. Epub 2015 Feb 26. J Mol Biol. 2015. PMID: 25728651 Free PMC article. Review.
-
The phagosomal solute transporter SLC15A4 promotes inflammasome activity via mTORC1 signaling and autophagy restraint in dendritic cells.EMBO J. 2022 Oct 17;41(20):e111161. doi: 10.15252/embj.2022111161. Epub 2022 Aug 29. EMBO J. 2022. PMID: 36031853 Free PMC article.
-
Lung Ischemia-Reperfusion is a Sterile Inflammatory Process Influenced by Commensal Microbiota in Mice.Shock. 2015 Sep;44(3):272-9. doi: 10.1097/SHK.0000000000000415. Shock. 2015. PMID: 26196836 Free PMC article.
References
-
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. - PubMed
-
- Kato H, Takahasi K, Fujita T. RIG-I-like receptors: cytoplasmic sensors for non-self RNA. Immunol. Rev. 2011;243:91–98. - PubMed
-
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell. 2002;10:417–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

