Permissive replication of homologous murine rotavirus in the mouse intestine is primarily regulated by VP4 and NSP1
- PMID: 23698306
- PMCID: PMC3719818
- DOI: 10.1128/JVI.00619-13
Permissive replication of homologous murine rotavirus in the mouse intestine is primarily regulated by VP4 and NSP1
Abstract
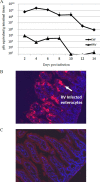
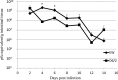
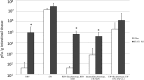
Homologous rotaviruses (RV) are, in general, more virulent and replicate more efficiently than heterologous RV in the intestine of the homologous host. The genetic basis for RV host range restriction is not fully understood and is likely to be multigenic. In previous studies, RV genes encoding VP3, VP4, VP7, nonstructural protein 1 (NSP1), and NSP4 have all been implicated in strain- and host species-specific infection. These studies used different RV strains, variable measurements of host range, and different animal hosts, and no clear consensus on the host range restriction determinants emerged. We used a murine model to demonstrate that enteric replication of murine RV EW is 1,000- to 10,000-fold greater than that of a simian rotavirus (RRV) in suckling mice. Intestinal replication of a series of EW × RRV reassortants was used to identify several RV genes that influenced RV replication in the intestine. The role of VP4 (encoded by gene 4) in enteric infection was strain specific. RRV VP4 reduced murine RV infectivity only slightly; however, a reassortant expressing VP4 from a bovine RV strain (UK) severely restricted intestinal replication in the suckling mice. The homologous murine EW NSP1 (encoded by gene 5) was necessary but not sufficient for promoting efficient enteric growth. Efficient enteric replication required a constellation of murine genes encoding VP3, NSP2, and NSP3 along with NSP1.
Figures
Similar articles
-
The Role of the VP4 Attachment Protein in Rotavirus Host Range Restriction in an In Vivo Suckling Mouse Model.J Virol. 2022 Aug 10;96(15):e0055022. doi: 10.1128/jvi.00550-22. Epub 2022 Jul 12. J Virol. 2022. PMID: 35862708 Free PMC article.
-
Roles of VP4 and NSP1 in determining the distinctive replication capacities of simian rotavirus RRV and bovine rotavirus UK in the mouse biliary tract.J Virol. 2011 Mar;85(6):2686-94. doi: 10.1128/JVI.02408-10. Epub 2010 Dec 29. J Virol. 2011. PMID: 21191030 Free PMC article.
-
Rotavirus NSP1 Contributes to Intestinal Viral Replication, Pathogenesis, and Transmission.mBio. 2021 Dec 21;12(6):e0320821. doi: 10.1128/mBio.03208-21. Epub 2021 Dec 14. mBio. 2021. PMID: 34903043 Free PMC article.
-
Silencing the alarms: Innate immune antagonism by rotavirus NSP1 and VP3.Virology. 2015 May;479-480:75-84. doi: 10.1016/j.virol.2015.01.006. Epub 2015 Feb 25. Virology. 2015. PMID: 25724417 Free PMC article. Review.
-
The Role of the Host Cytoskeleton in the Formation and Dynamics of Rotavirus Viroplasms.Viruses. 2024 Apr 25;16(5):668. doi: 10.3390/v16050668. Viruses. 2024. PMID: 38793550 Free PMC article. Review.
Cited by
-
Rhesus rotavirus NSP1 mediates extra-intestinal infection and is a contributing factor for biliary obstruction.PLoS Pathog. 2024 Sep 30;20(9):e1012609. doi: 10.1371/journal.ppat.1012609. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39348381 Free PMC article.
-
Serial Passaging of the Human Rotavirus CDC-9 Strain in Cell Culture Leads to Attenuation: Characterization from In Vitro and In Vivo Studies.J Virol. 2020 Jul 16;94(15):e00889-20. doi: 10.1128/JVI.00889-20. Print 2020 Jul 16. J Virol. 2020. PMID: 32461318 Free PMC article.
-
Characterization of rotavirus RNAs that activate innate immune signaling through the RIG-I-like receptors.PLoS One. 2013 Jul 23;8(7):e69825. doi: 10.1371/journal.pone.0069825. Print 2013. PLoS One. 2013. PMID: 23894547 Free PMC article.
-
VP4 Is a Determinant of Alpha-Defensin Modulation of Rotaviral Infection.J Virol. 2022 Apr 13;96(7):e0205321. doi: 10.1128/jvi.02053-21. Epub 2022 Mar 14. J Virol. 2022. Corrected and republished in: J Virol. 2023 Oct 31;97(10):e0096223. doi: 10.1128/jvi.00962-23 PMID: 35285683 Free PMC article. Corrected and republished.
-
Predicted structure and domain organization of rotavirus capping enzyme and innate immune antagonist VP3.J Virol. 2014 Aug;88(16):9072-85. doi: 10.1128/JVI.00923-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899176 Free PMC article.
References
-
- Estes M, Kapikian AZ. 2007. Rotaviruses, p 1917–1974 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA
-
- Ciarlet M, Schodel F. 2009. Development of a rotavirus vaccine: clinical safety, immunogenicity, and efficacy of the pentavalent rotavirus vaccine, RotaTeq. Vaccine 27(Suppl)6:G72–G81 - PubMed
-
- Ramig RF. 1988. The effects of host age, virus dose, and virus strain on heterologous rotavirus infection of suckling mice. Microb. Pathog. 4:189–202 - PubMed
-
- Ward LA, Rosen BI, Yuan L, Saif LJ. 1996. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J. Gen. Virol. 77(Pt 7):1431–1441 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

